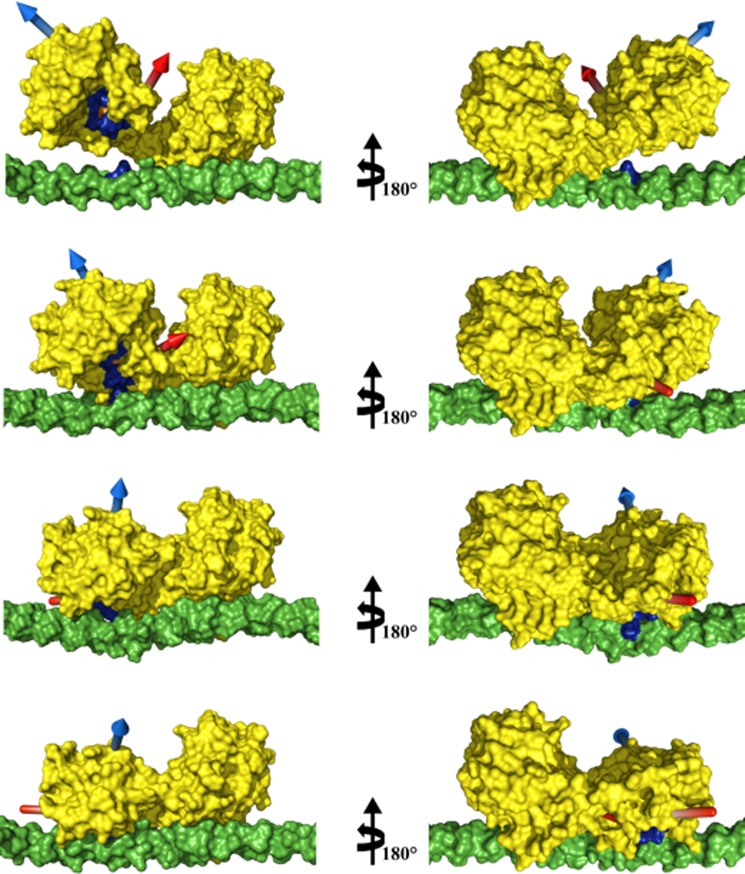
FIGURE 8.
Climber calculations of MMP-1 conformations. From top to bottom: structure with the highest MO, two morphing intermediate steps, and the previously proposed first step of collagenolysis (9). Structures in the right column are rotated 180° about the vertical axis with respect to the left column. The highest MO structure and morphing results were aligned to the HPX domain of the MMP-1·THP complex structure obtained previously (9). In yellow is the surface representation of MMP-1, in blue is the MMP consensus sequence HEXXHXXGXXH, in orange is the MMP-1 catalytic Zn2+, in green is the surface of the THP, and in blue is the THP cleavage site (Gly-Ile) of the first chain. The blue and red arrows indicate the directions of helices hA and hC, respectively, to facilitate visualizing the movement of the CAT domain with respect to the HPX domain and the THP. The THP sequence is (GPO)4-GPQGIAGQRGVVGLO-(GPO)4 (where O is 4-hydroxyproline), based on the human α1(I) collagen chain.