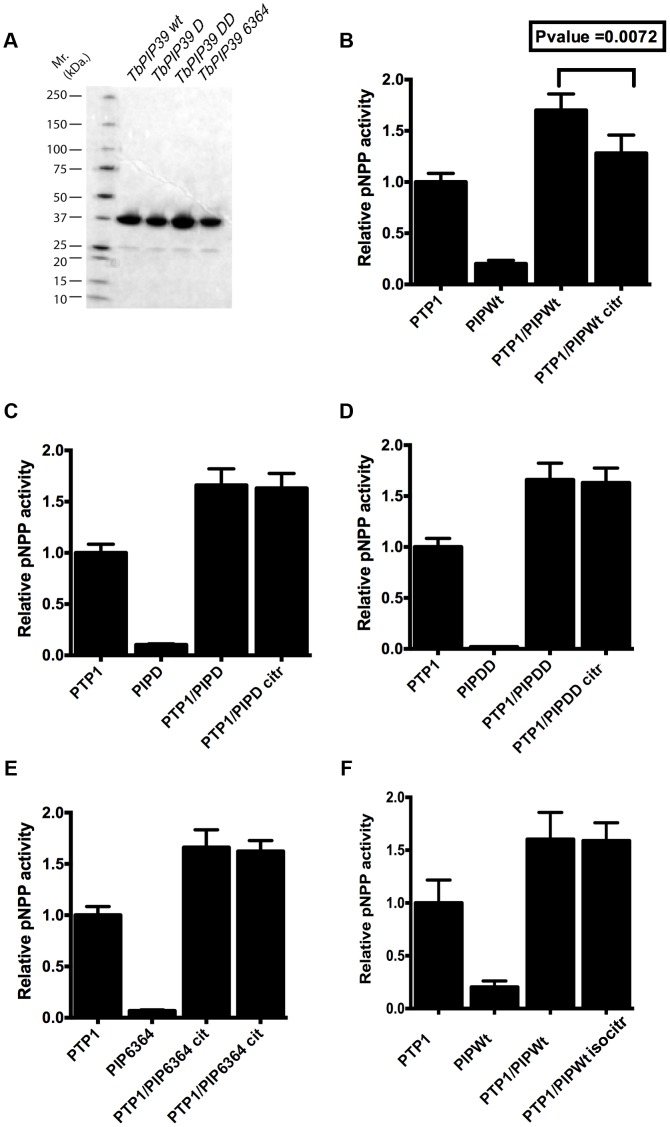
Figure 7. TbPIP39 mutants promote TbPTP1 activity but lack citrate responsiveness.
(A) Recombinant proteins of wild type and mutant TbPIP39 used for biochemical and biophysical analyses. (B)–(F) The protein phosphatase activity of TbPTP1 (0.1 µg) and various forms of TbPTP1 (1 µg of wild type, and the citrate binding mutants D, DD and 6364) were each measured. The combined pNPP activity of TbPTP1 and TbPIP39 were assayed in the absence and presence of citrate (2 mM) or, for the wild type TbPIP39, in the presence of 2 mM isocitrate, which is not a differentiation trigger (Panel F). In each case, TbPTP1 pNPP activity was normalised to 1.0 and all other pNPP activities were expressed relative to that. The error bars represent SD values of 5 independent triplicate assays. Only wild type TbPIP39 exhibited citrate-dependent inhibition of TbPTP1/TbPIP39 activity.