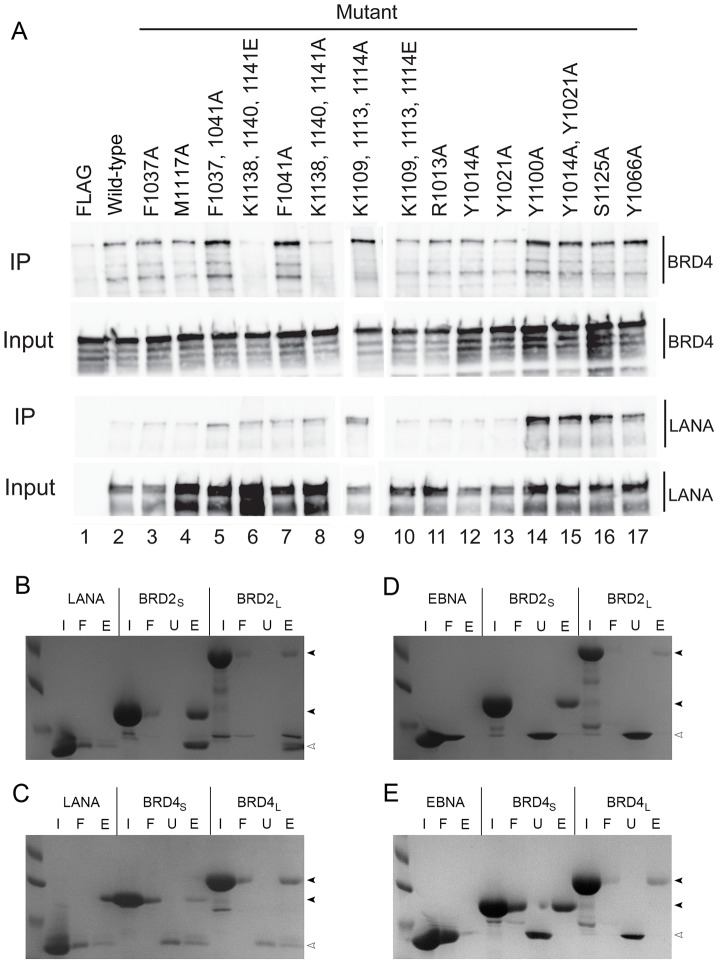
Figure 7. The basic patch of LANADBD interacts with the ET domain of BRD2 and BRD4.
(A) Wild-type LANA (lane 2) is able to co-immunoprecipitate BRD4 and mutations in the N-terminal arm (lanes 11–15), the tetrameric interface (lanes 3–5 and 7), and the interior portion of the basic patch (lanes 9–10) do not affect this interaction. However, mutation of Lys1138, Lys1140, and Lys1141 results in decreased levels of BRD4 interaction (lanes 6 and 8). IP and input are shown for BRD4 (top panels) and LANA (lower panels). (B–E) LANADBD is able to interact with BRD2 (B) and BRD4 (C) ET domains. Ni-NTA resin was loaded with LANA or His-tagged BRD ET domain (input, I) and the flow-through (F) was collected. Untagged LANA was then added and the unbound (U) fraction was collected. Complex formation is indicated by the presence of BRD (dark arrows) and LANA (open arrows) in the elution fraction (E). EBNA1DBD was not able to interact with either BRD2 or BRD4 ET domains (D, E).