Abstract
Q fever is serologically cross-reactive with other intracellular microorganisms. However, studies of the serological status of Mycoplasma pneumoniae and Chlamydophila pneumoniae during Q fever are rare. We conducted a retrospective serological study of M. pneumoniae and C. pneumoniae by enzyme-linked immunosorbent assay (ELISA), a method widely used in clinical practice, in 102 cases of acute Q fever, 39 cases of scrub typhus, and 14 cases of murine typhus. The seropositive (57.8%, 7.7%, and 0%, p<0.001) and seroconversion rates (50.6%, 8.8%, and 0%, p<0.001) of M. pneumoniae IgM, but not M. pneumoniae IgG and C. pneumoniae IgG/IgM, in acute Q fever were significantly higher than in scrub typhus and murine typhus. Another ELISA kit also revealed a high seropositivity (49.5%) and seroconversion rate (33.3%) of M. pneumoniae IgM in acute Q fever. The temporal and age distributions of patients with positive M. pneumoniae IgM were not typical of M. pneumoniae pneumonia. Comparing acute Q fever patients who were positive for M. pneumoniae IgM (59 cases) with those who were negative (43 cases), the demographic characteristics and underlying diseases were not different. In addition, the clinical manifestations associated with atypical pneumonia, including headache (71.2% vs. 81.4%, p=0.255), sore throat (8.5% vs. 16.3%, p=0.351), cough (35.6% vs. 23.3%, p=0.199), and chest x-ray suggesting pneumonia (19.3% vs. 9.5%, p=0.258), were unchanged between the two groups. Clinicians should be aware of the high seroprevalence of M. pneumoniae IgM in acute Q fever, particularly with ELISA kits, which can lead to misdiagnosis, overestimations of the prevalence of M. pneumoniae pneumonia, and underestimations of the true prevalence of Q fever pneumonia.
Introduction
Q fever, a zoonosis distributed worldwide, is caused by infection with the obligate intracellular microorganism Coxiella burnetii [1]. The primary animal reservoirs include cattle, sheep, and goats, and the major transmission route is human inhalation of aerosols or ingestion of unpasteurized dairy products contaminated with feces, urine, or reproductive tissues of infected animals. The clinical presentation of Q fever includes acute and chronic forms [2]. Acute Q fever presents with asymptomatic infection or influenza-like symptoms with various degrees of pneumonia or hepatitis. Culture-negative infective endocarditis is the major presentation of chronic Q fever [2]. During the largest outbreak of Q fever in the Netherlands, pneumonia (61.5%) was the most common presentation, and hepatitis accounted for only 0.4% [3].
Mycoplasma pneumoniae and Chlamydophila pneumoniae are common atypical pathogens of community-acquired pneumonia [4]. Due to a laborious and expensive culture procedure, serological assessment of antibodies is widely used to diagnose M. pneumoniae [5] and C. pneumoniae [6], with microimmunofluorescence (MIF) as the recommended method. However, because MIF is a time-consuming procedure and requires an experienced operator, the enzyme-linked immunosorbent assay (ELISA) has become the most commonly used method in clinical practice.
Because culture of C. burnetii is a laborious procedure and must be performed in a biosafety level 3 laboratory, serological assessment of antibodies against C. burnetii has become the gold-standard method for clinical diagnosis of Q fever. However, serological cross-reactivity with other intracellular pathogens, including Bartonella species [7,8], Legionella micdadei [9], Rickettsia species [10], Chlamydophila species [11], Mycoplasma pneumoniae [10], cytomegalovirus [10], and Epstein-Barr virus [10], has been reported in Q fever patients. Recently, a case of acute Q fever masquerading as M. pneumoniae pneumonia was reported [12]. In clinical practice, we have found several cases of acute Q fever that were serologically positive for M. pneumoniae or C. pneumoniae IgM before the final results of acute Q fever examinations were available. This finding indicates that patients can be misdiagnosed as atypical pneumonia if they are not tested for Q fever.
The aim of this study was to investigate the seroprevalence of antibodies against M. pneumoniae and C. pneumoniae, the two most common pathogens of atypical pneumonia, in patients with acute Q fever.
Methods
Selection of study cases
Because acute Q fever, scrub typhus (caused by Orientia tsutsugamushi), and murine typhus (caused by Rickettsia typhi) are the most common rickettsioses in Taiwan, and because they are difficult to differentiate from each other by clinical manifestations [13], cases confirmed for the 3 diseases were included in the investigation. From April 2004 to December 2009, a total of 166 cases of acute Q fever, scrub typhus, and murine typhus were diagnosed and confirmed by the Centers for Diseases Control of Taiwan (Taiwan CDC) at E-Da hospital. Among them, the sera of 160 cases were available for study. To clarify the seroprevalence of each disease, 5 previously published cases of acute Q fever and scrub typhus co-infections were excluded [14]. Finally, the sera (acute or convalescent phase) of 155 cases (102 acute Q fever, 39 scrub typhus, and 14 murine typhus) were included in the study. Among them, 135 cases (89 acute Q fever, 34 scrub typhus, and 12 murine typhus) had paired sera (acute and convalescent phase) for investigating the rates of antibody seroconversion.
Ethics Statement
This study was approved by the Ethics Committee of the E-Da Hospital (EMRP-097-117). The committee waived the need for written informed consent because the demographic information and clinical data were retrospectively recorded, and all of the data were collected anonymously.
Clinical characteristics and data collection
The demographic information, clinical manifestations, and results of laboratory and imaging examinations of the included cases were obtained retrospectively by medical chart review and were recorded using an anonymous case record form.
Confirmatory diagnosis of acute Q fever, scrub typhus, and murine typhus
Serological tests for the presence of specific antibodies against C. burnetii, O. tsutsugamushi, and R. typhi were performed using an indirect immunofluorescence antibody assay (IFA) in the contract laboratory of the Taiwan CDC, as previously described [13]. Acute Q fever was diagnosed either by an anti-phase II antigen IgG titer of ≥ 1:320 and an anti-phase II antigen IgM titer of ≥ 1:80, a four-fold or greater increase of anti-phase II antigen IgG titer in paired sera, or by blood that tested positive for C. burnetii DNA by polymerase chain reaction (PCR) [15]. Scrub typhus and murine typhus were diagnosed by a specific antibody titer of IgM ≥ 1:80, a four-fold or greater rise of IgG titer in paired sera, or by blood that tested positive for O. tsutsugamushi and R. typhi DNA by PCR, respectively.
Detection of serum antibodies against Mycoplasma pneumoniae and Chlamydophila pneumoniae
Serum was the residual specimen obtained for the diagnosis-related purposes of rickettsioses for the Taiwan CDC, and it was stored at -80°C until analysis. The serum IgG and IgM antibodies against M. pneumoniae and C. pneumoniae were detected using commercially available ELISA kits of M. pneumoniae IgG (MYCG0350)/IgM (MYCM0350) and C. pneumoniae IgG (CHLG0510)/IgM (CHLM0510) (ELISA kits, NovaLisa™, NovaTec Immundiagnostica GmbH, Germany), respectively. These kits were used for the detection of serum M. pneumoniae and C. pneumoniae IgG or IgM in clinical practice at E-Da Hospital. To validate the results, a second commercial ELISA kit for the detection of M. pneumoniae IgG (SeroMPTM IgG)/IgM (SeroMPTM IgM) and C. pneumoniae IgG (SeroCPTM IgG)/IgM (SeroCPTM IgM) (ELISA kits, Savyon Diagnostics, Ashdod, Israel) was used. All of the procedures and the interpretation of antibody determinations were performed according to the manufacturer's instructions, and each examination was performed in duplicate.
Statistical analysis
Categorical variables were analyzed using the Chi-square or Fisher’s exact test where appropriate. Continuous variables were analyzed using Student’s t-test. All p values were two-tailed, and a p value <0.05 was considered to be statistically significant. The data were analyzed with SPSS software for Windows (Release 15.0; SPSS, Chicago, IL).
Results
Overall status of M. pneumoniae and C. pneumoniae IgG/IgM in acute Q fever, scrub typhus, and murine typhus
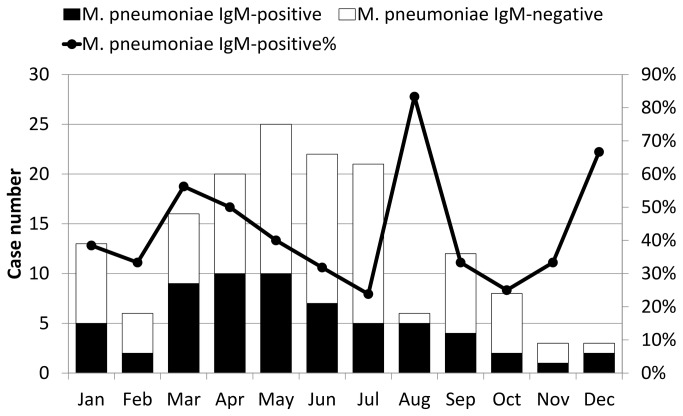
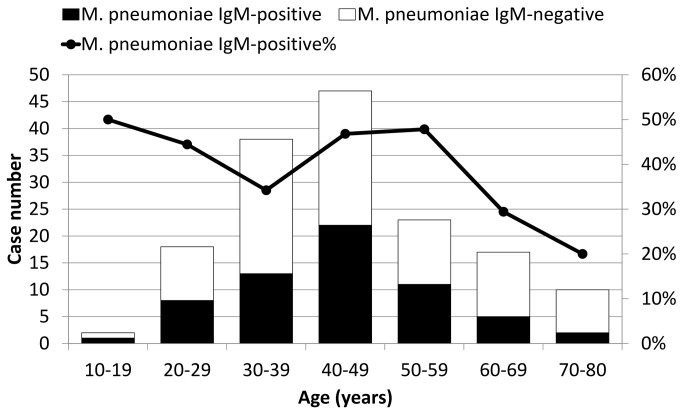
The serum IgG or IgM results of M. pneumoniae and C. pneumoniae in the 155 cases using the first ELISA kits are shown in Table 1. In acute or convalescent phase sera, 62 (40.0%) and 43 cases (27.7%) had M. pneumoniae IgM and C. pneumoniae IgM, respectively. The monthly distribution of serum positive cases of M. pneumoniae IgM is shown in Figure 1, and they reached peaks in March, August, and December. Figure 2 illustrates the age distribution of the serum positive cases of M. pneumoniae IgM, and it was highest for those aged between 40 and 59 years old. The positivity rates of M. pneumoniae IgM in the acute (13.9%, 0%, and 0%, p=0.012), convalescent (58.9%, 8.8%, and 0%, p<0.001), and acute or convalescent phases (57.8%, 7.7%, and 0%, p<0.001) of acute Q fever were significantly higher than scrub typhus or murine typhus (Table 1). In contrast, the positivity rates of M. pneumoniae IgG and C. pneumoniae IgG/IgM were not different between the 3 diseases. To further investigate the seroconversion of M. pneumoniae and C. pneumoniae antibodies at the acute and convalescent phases, the 135 patients with available paired sera were analyzed, and the results are shown in Table 2. The seroconversion rate of M. pneumoniae IgM in acute Q fever was significantly higher than scrub typhus or murine typhus (50.6%, 8.8%, and 0%, p<0.001). However, the seroconversion rates of M. pneumoniae IgG and C. pneumoniae IgG/IgM were not different. Among the 56 acute Q fever patients with serum M. pneumoniae IgM, only 2 (3.6%) and 16 (28.6%) had 4-fold increases of IgG and IgG seroconversion, respectively (Table 3). Taken together, only the positivity and seroconversion rates of M. pneumoniae IgM in acute Q fever were significantly higher than scrub typhus or murine typhus.
Table 1. The results of all available sera tested for Mycoplasma pneumoniae and Chlamydophila pneumoniae IgM and IgG antibodies a in patients with acute Q fever, scrub typhus, and murine typhus.
| Positive antibodies in the acute or convalescent phase | Acute Q fever (N=102) | Scrub typhus (N=39) | Murine typhus (N=14) | Total (n=155) | p f |
|---|---|---|---|---|---|
| Mycoplasma pneumoniae IgMb | |||||
| Acute or convalescent phase | 59/102 (57.8) | 3/39 (7.7) | 0/14 (0) | 62/155 (40.0) | <0.001 |
| Acute phase | 14/101 (13.9) | 0/39 (0) | 0/13 (0) | 14/153 (9.2) | 0.012 |
| Convalescent phase | 53/90 (58.9) | 3/34 (8.8) | 0/13 (0) | 56/137 (40.9) | <0.001 |
| Mycoplasma pneumoniae IgGc | |||||
| Acute or convalescent phase | 72/102 (70.6) | 22/39 (56.4) | 9/14 (64.3) | 103/155 (66.5) | 0.274 |
| Acute phase | 51/101 (50.5) | 14/39 (35.9) | 6/13 (46.2) | 71/153 (46.4) | 0.308 |
| Convalescent phase | 63/90 (70.0) | 17/34 (50.0) | 7/13 (53.8) | 87/137 (63.5) | 0.092 |
| Chlamydophila pneumoniae IgMd | |||||
| Acute or convalescent phase | 26/102 (25.5) | 11/39 (28.2) | 6/14 (42.9) | 43/155 (27.7) | 0.357 |
| Acute phase | 11/101(10.9) | 6/39 (15.4) | 2/13 (15.4) | 19/153 (12.4) | 0.671 |
| Convalescent phase | 22/90 (24.4) | 10 /34(29.4) | 6/13 (46.2) | 38/137 (27.7) | 0.279 |
| Chlamydophila pneumoniae IgGe | |||||
| Acute or convalescent phase | 67/102 (65.7) | 31/39 (79.5) | 11/14 (78.6) | 109/155 (70.3) | 0.257 |
| Acute phase | 47/101 (46.5) | 23/39 (59.0) | 8/13 (61.5) | 78/153 (51.0) | 0.326 |
| Convalescent phase | 55/90 (61.1) | 26/34 (76.5) | 10/13 (76.9) | 91/137 (66.4) | 0.197 |
Tested by ELISA (NovaTec Immundiagnostica GmbH, Germany).
Positive threshold for M. pneumoniae IgM is > 11 NTU. The positive values ranged from 11.3 to 46.9 (median=15.6) NTU.
Positive threshold for M. pneumoniae IgG is > 11 NTU. The positive values ranged from 11.1 to 47.9 (median=17.0) NTU.
Positive threshold for C. pneumoniae IgM is > 11 NTU (NovaTec-Units). The positive values ranged from 11.1 to 36.2 (median=15.7) NTU.
Positive threshold for C. pneumoniae IgG is > 11 NTU. The positive values ranged from 11.1 to 63.1 (median=20.3) NTU.
Chi-square or Fisher’s exact test between acute Q fever, scrub typhus, and murine typhus.
Figure 1. Monthly distribution of 62 cases with either acute or convalescent phase Mycoplasma pneumoniae IgM sera, 155 cases of acute Q fever (59 of 102 cases, 57.8%), scrub typhus (3 of 39 cases, 7.7%), and murine typhus (0 of 14 cases, 0%).
Figure 2. Age distribution of 62 cases with either acute or convalescent phase Mycoplasma pneumoniae IgM sera, 155 cases of acute Q fever (59 of 102 cases, 57.8%), scrub typhus (3 of 39 cases, 7.7%), and murine typhus (0 of 14 cases, 0%).
Table 2. The results of paired sera tested for Mycoplasma pneumoniae and Chlamydophila pneumoniae IgM and IgG antibodies a in patients with acute Q fever, scrub typhus, and murine typhus.
| Antibodies in the acute and convalescent phase | Acute Q fever (N=89) | Scrub typhus (N=34) | Murine typhus (N=12) | Total (N=135) | p f |
|---|---|---|---|---|---|
| Mycoplasma pneumoniae IgMb | |||||
| Seroconversion | 45 (50.6) | 3 (8.8) | 0 (0) | 48 (35.6) | <0.001 |
| Seroreversion | 3 (3.4) | 0 (0) | 0 (0) | 3 (2.2) | 0.668 |
| Both positive | 8 (9.0) | 0 (0) | 0 (0) | 8 (5.9) | 0.151 |
| Both negative | 33 (37.1) | 31 (91.2) | 12 (100) | 76 (56.3) | <0.001 |
| Mycoplasma pneumoniae IgGc | |||||
| Seroconversion | 21 (23.6) | 8 (23.5) | 2 (16.7) | 31 (23.0) | 0.950 |
| Seroreversion | 1 (1.1) | 2 (5.9) | 2 (16.7) | 5 (3.7) | 0.018 |
| Both positive | 42 (47.2) | 9 (26.5) | 4 (33.3) | 55 (40.7) | 0.093 |
| Both negative | 25 (28.1) | 15 (44.1) | 4 (33.3) | 44 (32.6) | 0.245 |
| Chlamydophila pneumoniae IgMd | |||||
| Seroconversion | 15 (16.9) | 5 (14.7) | 4 (33.3) | 24 (17.8) | 0.372 |
| Seroreversion | 3 (3.4) | 1 (2.9) | 0 (0) | 4 (3.0) | 0.999 |
| Both positive | 7 (7.9) | 5 (14.7) | 2 (16.7) | 14 (10.4) | 0.313 |
| Both negative | 64 (71.9) | 23 (67.6) | 6 (50.0) | 93 (68.9) | 0.287 |
| Chlamydophila pneumoniae IgGe | |||||
| Seroconversion | 19 (21.3) | 8 (23.5) | 2 (16.7) | 29 (21.5) | 0.947 |
| Seroreversion | 7 (7.9) | 3 (8.8) | 0 (0) | 10 (7.4) | 0.776 |
| Both positive | 35 (39.3) | 18 (52.9) | 7 (58.3) | 60 (44.4) | 0.240 |
| Both negative | 28 (31.5) | 5 (14.7) | 3 (25.0) | 36 (26.7) | 0.160 |
Tested by ELISA (NovaTec Immundiagnostica GmbH, Germany).
Positive threshold for M. pneumoniae IgM is > 11 NTU (NovaTec-Units). The positive values ranged from 11.3 to 46.9 (median=15.8) NTU.
Positive threshold for M. pneumoniae IgG is > 11 NTU. The positive values ranged from 11.1 to 47.9 (median=17.3) NTU.
Positive threshold for C. pneumoniae IgM is > 11 NTU. The positive values ranged from 11.1 to 36.2 (median=15.8) NTU.
Positive threshold for C. pneumoniae IgG is > 11 NTU. The positive values ranged from 11.1 to 63.1 (median=21.3) NTU.
Chi-square or Fisher’s exact test between acute Q fever, scrub typhus, and murine typhus.
Table 3. Four-fold increases and seroconversion of M. pneumoniae IgG in acute Q fever with serum M. pneumoniae IgM by 2 ELISA kits.
| ELISA kit | 4-fold increase of M. pneumoniae IgG | Seroconversion of M. pneumoniae IgG |
|---|---|---|
| NovaTec Immundiagnostica GmbH, Germany | 3.6% (2/56)a | 28.6% (16/56)b |
| Savyon Diagnostics, Ashdod, Israel | 11.1% (5/45)c | 11.1% (5/45)c |
One of the two patients had seroconversion of M. pneumoniae IgG
None of the 16 patients had 4-fold increases of M. pneumoniae IgG
All of the 5 patients had 4-fold increases and seroconversion of M. pneumoniae IgG
Results of M. pneumoniae and C. pneumoniae IgG/IgM in acute Q fever with the second ELISA kit
The second ELISA kits (Savyon Diagnostics, Ashdod, Israel) were used to validate the high seropositivity rate of M. pneumoniae IgM found in acute Q fever cases. The results and concordant rates compared to the first ELISA kits are listed in Table 4. These revealed positivity rates of 21% (21/100), 45.3% (39/86), and 49.5% (51/103) during the acute, convalescent, and acute or convalescent phases, respectively. Among the cases with available paired sera, the seroconversion rate of M. pneumoniae IgM was 33.3% (28/84). Various degrees of concordant rates were found when compared to the first ELISA kits. The 4-fold increase of IgG and IgG seroconversion in the 45 acute Q fever patients with serum M. pneumoniae IgM were both 11.1% (Table 3).
Table 4. The results and concordant rates of Mycoplasma pneumoniae and Chlamydophila pneumoniae IgM and IgG antibodies in patients with acute Q fever tested by ELISA (Savyon Diagnostics, Ashdod, Israel).
| Antibodies in the acute and/or convalescent phase | Positive number/ tested number (%) | Concordant ratese compared with ELISA kits (NovaTec Immundiagnostica GmbH, Germany) |
|---|---|---|
| Mycoplasma pneumoniae IgMa | ||
| Acute phase | 21/100 (21) | 52.4% |
| Convalescent phase | 39/86 (45.3) | 79.5% |
| Acute or convalescent phase | 51/103 (49.5) | 72.5% |
| Seroconversion | 28/84 (33.3) | 67.9% |
| Mycoplasma pneumoniae IgGb | ||
| Acute phase | 18/99 (18.2) | 94.4% |
| Convalescent phase | 47/89 (52.8) | 87.2% |
| Acute or convalescent phase | 50/102 (49.0) | 88.0% |
| Seroconversion | 30/86 (34.9) | 30.0% |
| Chlamydophila pneumoniae IgMc | ||
| Acute phase | 9/97 (9.3) | 55.6% |
| Convalescent phase | 29/86 (33.7) | 27.6% |
| Acute or convalescent phase | 34/101 (33.7) | 38.2% |
| Seroconversion | 23/82 (28.0) | 21.7% |
| Chlamydophila pneumoniae IgGd | ||
| Acute phase | 57/98 (58.2) | 73.7% |
| Convalescent phase | 52/82 (63.4) | 78.8% |
| Acute or convalescent phase | 73/100 (73.0) | 78.1% |
| Seroconversion | 14/80 (17.5) | 35.7% |
Positive threshold for M. pneumoniae IgM is > 20 BU/ml). The positive values ranged from 20.6 to 146.3 (median=40.7) BU/ml.
Positive threshold for M. pneumoniae IgG is > 20 BU/ml. The positive values ranged from 20.1 to 163.8 (median=38.7) BU/ml.
Positive threshold for C. pneumoniae IgM is > 1.5 COI. The positive values ranged from 1.51 to 5.25 (median=2.0) COI.
Positive threshold for C. pneumoniae IgG is > 1.1 COI. The positive values ranged from 11.1 to 6.31 (median=2.06) COI.
Concordant rate = number of positive samples by both kits/number of positive samples by each ELISA kit (Savyon Diagnostics, Ashdod, Israel) x 100
Status of M. pneumoniae IgM and clinical manifestations of acute Q fever
To investigate the possible clinical differences between acute Q fever with and without serum M. pneumoniae IgM, the 102 cases of acute Q fever listed in Table 1 were divided into 2 groups according to the results of the M. pneumoniae IgM in acute or convalescent phase serum for comparison (59 positive cases [57.8%] and 43 negative cases [42.2%]). There were no significant differences among demographic data, underlying diseases, or clinical symptoms and signs between the 2 groups of patients (Table 5). It is noteworthy that symptoms possibly associated with atypical pneumonia, such as headache (71.2% vs. 81.4%, p=0.255), sore throat (8.5% vs. 16.3%, p=0.351), and cough (35.6% vs. 23.3%, p=0.199), were not different. In imaging findings, laboratory examinations, and responses to treatment, there were no differences between the patients with and without serum M. pneumoniae IgM (Table 6). The rate of chest x-ray abnormalities, possibly suggesting pneumonia, was likewise unchanged (19.3% vs. 9.5%, p=0.258). Similarly, no significant differences were found by comparing the same variables listed in Tables 5 and 6 between patients with and without seroconversion of M. pneumoniae IgM (data not shown).
Table 5. Differences among demographic data, underlying diseases, and clinical symptoms and signs between acute Q fever patients whose sera were positive and negative for Mycoplasma pneumoniae IgMa.
| Clinical characteristics | Negative for M. pneumoniae IgM (N=43) | Positive for M. pneumoniae IgM (N=59) | Total (N=102) | p b |
|---|---|---|---|---|
| Demographic data and underlying diseases | ||||
| Male gender | 40 (93.0) | 55(93.2) | 95 (93.1) | 0.999 |
| Age (years)c | 43.6 ± 11.8 | 44.8 ± 12.0 | 44.3 ± 11.9 | 0.602 |
| HBV or HCV infectiond | 11 (25.6) | 21 (35.6) | 32 (31.4) | 0.388 |
| Liver cirrhosis | 1 (2.3) | 0 (0) | 1 (1.0) | 0.422 |
| Hypertension | 4 (9.3) | 7 (11.9) | 11 (10.8) | 0.757 |
| Diabetes mellitus | 4 (9.3) | 3 (5.1) | 7 (6.9) | 0.451 |
| Congestive heart failure | 0 (0) | 1 (1.7) | 1 (1.0) | 0.999 |
| COPD | 0 (0) | 1 (1.7) | 1 (1.0) | 0.999 |
| Malignancy | 0 (0) | 2 (3.4) | 2 (2.0) | 0.507 |
| HIV infection | 1 (2.3) | 0 (0) | 1 (1.0) | 0.422 |
| Clinical symptoms and signs | ||||
| Fever | 42 (97.7) | 59 (100) | 101 (99.0) | 0.422 |
| Chills | 34 (79.1) | 50 (84.7) | 84 (82.4) | 0.600 |
| Headache | 35 (81.4) | 42 (71.2) | 77 (75.5) | 0.255 |
| Sore throat | 7 (16.3) | 5 (8.5) | 12 (11.8) | 0.351 |
| Jaundice | 2 (4.7) | 3 (5.1) | 5 (4.9) | 0.999 |
| Cough | 10 (23.3) | 21 (35.6) | 31 (30.4) | 0.199 |
| Nausea or vomiting | 2 (4.7) | 4 (6.8) | 6 (5.9) | 0.999 |
| Abdominal pain or discomfort | 7 (16.3) | 6 (10.2) | 13 (12.7) | 0.384 |
| Diarrhea | 4 (9.3) | 5 (8.5) | 9 (8.8) | 0.999 |
| General weakness | 8 (18.6) | 5 (8.5) | 13 (12.7) | 0.145 |
| Arthralgia | 1 (2.3) | 2 (3.4) | 3 (2.9) | 0.999 |
| Myalgia | 19 (44.2) | 18 (30.5) | 37 (36.3) | 0.211 |
| Relative bradycardiae | 16 (37.2) | 32 (54.2) | 48 (47.1) | 0.110 |
Tested by ELISA (NovaTec Immundiagnostica GmbH, Germany).
Categorical variables were analyzed using the Chi-square or Fisher’s exact test as appropriate. Continuous variables were analyzed using Student’s t-test.
Presented as the mean value ± standard deviation.
Confirmed by examinations of HBsAg and anti-HCV.
Body temperature ≥ 38.9oC and heart rate < 110/min without medication with calcium blockers, beta-blockers, or anti-arrhythmic agents.
Table 6. Differences among imaging findings, laboratory examinations, and responses to treatment between acute Q fever patients whose sera were positive and negative for Mycoplasma pneumoniae IgMa.
| Clinical characteristics | Negative for M. pneumoniae IgM (N=43) | Positive for M. pneumoniae IgM (N=59) | Total (N=102) | p b |
|---|---|---|---|---|
| Abdominal sonography or computed tomography findings | 39 (90.7) | 56 (94.9) | 0.451 | |
| Days from disease onsetc | 6.8 ± 4.0 | 7.0 ± 3.3 | 6.9 ± 3.6 | 0.778 |
| Abnormal | 18/39 (46.2) | 25/56 (44.6) | 43/95 (45.3) | 0.999 |
| Hepatomegaly or splenomegaly | 15 (38.5) | 18 (32.1) | 33/95 (34.7) | 0.662 |
| Hepatomegaly | 9/39 (23.1) | 13/56 (23.2) | 22/95 (23.2) | 0.999 |
| Splenomegaly | 12/39 (30.8) | 10/56 (17.9) | 22/95 (23.2) | 0.216 |
| Cholecystitic change | 6/39 (15.4) | 17/56 (30.4) | 23/95 (24.2) | 0.143 |
| Fatty liver | 22/39 (56.4) | 28/56 (50.0) | 50/95 (52.6) | 0.676 |
| Chest x-ray | ||||
| Days from disease onsetc | 5.3 ± 3.1 | 5.7 ± 2.7 | 5.5 ± 2.9 | 0.519 |
| Abnormal CXR finding | 4/42 (9.5) | 11/57 (19.3) | 16/99 (15.2) | 0.258 |
| Unilateral infiltration | 2/42 (4.8) | 5/57 (8.8) | 7/99 (7.1) | 0.695 |
| Bilateral infiltration | 2/42 (4.8) | 6/57 (10.5) | 8/99 (8.1) | 0.461 |
| Pneumonia patch | 0/42 (0) | 0/57 (0) | 0/99 (0) | NC |
| Complete blood cell examination | ||||
| Days from disease onsetc | 5.1 ± 3.1 | 5.3 ± 2.5 | 5.2 ± 2.7 | 0.813 |
| Leukocytosis | 2 (4.7) | 2 (3.4) | 4/102 (3.9) | 0.999 |
| Leukopenia | 9 (20.9) | 7 (11.9) | 16/102 (15.7) | 0.273 |
| Anemia | 0 (0) | 0 (0) | 0/102 (0) | NC |
| Thrombocytopenia | 30 (69.8) | 43 (72.9) | 73/102 (71.6) | 0.825 |
| Liver transaminase | ||||
| Days from disease onsetc | 5.6 ± 3.9 | 5.7 ± 2.6 | 5.6 ± 3.2 | 0.849 |
| GPT > 88 | 23/42 (54.8) | 42/59 (71.2) | 65/101 (64.4) | 0.097 |
| GOT > 76 | 27/42 (64.3) | 43/58 (74.1) | 70/100 (70.0) | 0.377 |
| GPT > 44 | 40/42 (95.2) | 58/59 (98.3) | 98/101 (97.0) | 0.569 |
| GOT > 38 | 41/42 (97.6) | 57/58 (98.3) | 98/100 (98.0) | 0.999 |
| Doxycycline treatment to defervescence > 3 days | 5/35 (14.3) | 10/44 (22.7) | 15/79 (19.0) | 0.398 |
Tested by ELISA (NovaTec Immundiagnostica GmbH, Germany).
Categorical variables were analyzed using the Chi-square or Fisher’s exact test as appropriate. Continuous variables were analyzed using Student’s t-test.
Presented as the mean value ± standard deviation.
Discussion
Various degrees of sensitivity, specificity, and cross-reactivity were reported in several commercially available kits of M. pneumoniae [16-19] and C. pneumoniae [20,21], but the sera from rickettsioses were rarely used as controls for the evaluation of specificity [19]. In the present study, 57.8% and 25.5% of acute Q fever, 7.7% and 28.2% of scrub typhus, and 0% and 42.9% of murine typhus cases were serum positive for M. pneumoniae IgM and C. pneumoniae IgM, respectively (Table 1). The seropositive rates of M. pneumoniae IgM in acute Q fever were much higher than the acute infection rates of M. pneumoniae as determined by serological studies in healthy adolescents (6.0%) [22], adults with respiratory symptoms (3.3%) [23], and community-acquired pneumonia (CAP) (14.3%-20.0%) [24,25] in Taiwan. The seropositivity rates of C. pneumoniae IgM were also higher than the percentage of C. pneumoniae pneumonia in CAP in Taiwan (7.1%-13.0%) [24,25]. Accordingly, the high seropositivity rates of M. pneumoniae and C. pneumoniae IgM in the present study did not result from background seroprevalence. These results had two important impacts. First, in clinical practice, rickettsioses may be misdiagnosed as atypical pneumonia if only M. pneumoniae and C. pneumoniae antibodies are tested. Second, in the investigation of M. pneumoniae and C. pneumoniae ELISA kits, the sera from rickettsioses should be included for evaluating specificity and possible false-positivity of the ELISA kits.
Both Q fever pneumonia and M. pneumoniae pneumonia belong to atypical pneumonia, but serological studies for one in the other are rare [10,12,19]. A case of Q fever presented with acalculous cholecystitis was reported to have serum M. pneumoniae IgM (SeroMPTM) [12]. Vardi et al. reported that 4 of 33 (12.1%) acute Q fever cases had serum M. pneumoniae IgM [10]. In a study by Beersma et al., 3 of the 12 serological kits for M. pneumoniae had false positivity for M. pneumoniae IgM in 4 Q fever controls (ImmunoCardTM [75%, 3/4], NovumTM [100%, 4/4], SeroMPTM [50%, 2/4]) [19]. In the clinical setting, possible dual infections of C. burnetii and M. pneumoniae or C. pneumoniae have rarely been reported in studies of CAP [26,27]. In an 11-year study conducted in the Canary Islands, 10% of CAP cases were mixed infections. C. burnetii was the most frequently isolated co-pathogen, but the pathogens were not clearly described [26]. In a study in northern Israel, Shibli et al. identified Q fever as the microbiological etiology of 8 CAP cases in which 3 (37.5%) and 2 (25.0%) were combined infections with C. pneumoniae and M. pneumoniae, respectively [27]. In clinical interpretations, the seropositivity and seroconversion of M. pneumoniae IgM suggests a recent infection, which indicates that as many as 50-60% acute Q fever cases could be diagnosed as M. pneumoniae infection without testing for Q fever. From the epidemiological perspective, this would underestimate and overestimate the true prevalence of Q fever and M. pneumoniae infections, respectively.
Acute Q fever, followed by scrub typhus and murine typhus, is the most common rickettsiosis, and it is an emerging and endemic disease in southern Taiwan [13]. In Taiwan, hepatitis rather than pneumonia is the predominant presentation of acute Q fever [28,29]. In our previous study, although all of the cases of acute Q fever had hepatitis, 19% of them had abnormal chest x-ray findings, and 9.5% had unilateral and 9.5% bilateral infiltration [29]. This result indicated that nearly 20% of acute Q fever cases might be diagnosed and treated as pneumonia based on chest x-ray findings without testing for Q fever. In the present study, 57.8% and 25.5% of acute Q fever cases had serum M. pneumoniae IgM and C. pneumoniae, respectively (Table 1), and 19.3% of cases with M. pneumoniae IgM had abnormalities suggestive of pneumonia by chest x-ray (Table 6). These findings highlight that, before the confirmatory results of Q fever are available or without testing for Q fever, approximately 60% and 25% of cases may be misdiagnosed as M. pneumoniae and C. pneumoniae infection, respectively. Conversely, acute Q fever may account for a portion of serologically diagnosed M. pneumoniae and C. pneumoniae pneumonia, and the true prevalence of acute Q fever presenting with pneumonia in southern Taiwan may be underestimated.
Several points suggest that the high seropositivity rate of M. pneumoniae IgM in acute Q fever may be due to serological cross-reactivity rather than true M. pneumoniae infection. First, in addition to developing auto-antibodies [10,30,31], serological cross-reactivity with other pathogens is common in Q fever, including M. pneumoniae [7-11]. Second, the risk of dual infections of non-zootic (M. pneumoniae) and zoonotic (C. burnetii) atypical pneumonia is low [32]. Third, the monthly and age distributions (Figures 1 and 2) were not typical for M. pneumoniae pneumonia, which generally predominates in autumn among children and young adults [33]. Fourth, the clinical symptoms and chest-x ray findings possibly associated with M. pneumoniae pneumonia were not different between acute Q fever patients with and without serum M. pneumoniae IgM (Tables 5 and 6). Fifth, it is unreasonable that patients with acute Q fever rather than scrub typhus or murine typhus tend to contract dual infections with M. pneumoniae. From the viewpoint of the immune response to M. pneumoniae, despite high seropositivity (57.8%) (Table 1) and seroconversion rates of M. pneumoniae IgM (50.6%) (Table 2) in acute Q fever, a high IgG seropositivity rate during the acute phase (50.5%), a low IgG seroconversion rate (23.6%), and various concordant rates between the 2 ELISA kits (Table 4) are not typical serological patterns of acute M. pneumoniae infection. Further investigation of acute Q fever patients with M. pneumoniae IgM revealed that few had 4-fold increases (3.6% and 11.1%) and IgG seroconversion (28.6% and 11.1%) as measured by the two ELISA kits (Table 3), indicating that the presence of IgM did not result from true infection. However, the hypothesis of cross-reactivity between acute Q fever serum and M. pneumoniae IgM ELISA kits requires determination by cross-adsorption studies and Western blot analysis, which require culture and large quantities of C. burnetii and M. pneumoniae antigens in a professional research laboratory [7,9,34].
This retrospective clinical and serological study has several limitations. We only tested 2 ELISA kits commonly used in Taiwan for the serological diagnosis of M. pneumoniae and C. pneumoniae infections. Whether a similar phenomenon exists with other kits needs further investigation. The studied cases were mainly distributed across southern Taiwan, and the results require a large-scale study involving other countries to be confirmed. The hypothesis of serological cross-reactivity derived from clinical observations needs further investigation by basic medical research in a professional laboratory.
In conclusion, nearly 60% of acute Q fever cases were serum positive for M. pneumoniae IgM by ELISA. However, their clinical manifestations were not different from those that were serum negative for M. pneumoniae IgM. In addition, approximately 25% of acute Q fever cases were serum positive for C. pneumoniae IgM. Clinicians should be aware of the high seroprevalence of M. pneumoniae IgM, particularly using ELISA kits, in acute Q fever. This observation could lead to misdiagnosis, overestimation of the prevalence of M. pneumoniae infection, and underestimations of the true prevalence of Q fever pneumonia.
Funding Statement
This work was supported by research grants from the National Science Council [NSC 99-2745-B-650-001] and E-Da Hospital [EDAHP100001]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maurin M, Raoult D (1999) Q fever. Clin Microbiol Rev 12: 518-553. PubMed: 10515901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raoult D, Marrie T, Mege J (2005) Natural history and pathophysiology of Q fever. Lancet Infect Dis 5: 219-226. doi: 10.1016/S1473-3099(05)70052-9. PubMed: 15792739. [DOI] [PubMed] [Google Scholar]
- 3. Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A et al. (2012) The 2007-2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol 64: 3-12. doi: 10.1111/j.1574-695X.2011.00876.x. PubMed: 22066649. [DOI] [PubMed] [Google Scholar]
- 4. Arnold FW, Summersgill JT, Lajoie AS, Peyrani P, Marrie TJ et al. (2007) A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 175: 1086-1093. doi: 10.1164/rccm.200603-350OC. PubMed: 17332485. [DOI] [PubMed] [Google Scholar]
- 5. Atkinson TP, Balish MF, Waites KB (2008) Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev 32: 956-973. doi: 10.1111/j.1574-6976.2008.00129.x. PubMed: 18754792. [DOI] [PubMed] [Google Scholar]
- 6. Kumar S, Hammerschlag MR (2007) Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis 44: 568-576. doi: 10.1086/511076. PubMed: 17243062. [DOI] [PubMed] [Google Scholar]
- 7. La Scola B, Raoult D (1996) Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii . J Clin Microbiol 34: 2270-2274. PubMed: 8862597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graham JV, Baden L, Tsiodras S, Karchmer AW (2000) Q fever endocarditis associated with extensive serological cross-reactivity. Clin Infect Dis 30: 609-610. doi: 10.1086/313701. PubMed: 10722459. [DOI] [PubMed] [Google Scholar]
- 9. Musso D, Raoult D (1997) Serological cross-reactions between Coxiella burnetii and Legionella micdadei . Clin Diagn Lab Immunol 4: 208-212. PubMed: 9067657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vardi M, Petersil N, Keysary A, Rzotkiewicz S, Laor A et al. (2011) Immunological arousal during acute Q fever infection. Eur J Clin Microbiol Infect Dis 30: 1527-1530. doi: 10.1007/s10096-011-1255-5. PubMed: 21509477. [DOI] [PubMed] [Google Scholar]
- 11. Lukácová M, Melnicáková J, Kazár J (1999) Cross-reactivity between Coxiella burnetii and Chlamydiae . Folia Microbiol (Praha) 44: 579-584. doi: 10.1007/BF02816263. PubMed: 10997139. [DOI] [PubMed] [Google Scholar]
- 12. Lee CH, Chuah SK, Pei SN, Liu JW (2011) Acute Q fever presenting as antiphospholipid syndrome, pneumonia, and acalculous cholecystitis and masquerading as Mycoplasma pneumoniae and hepatitis C viral infections. Jpn J Infect Dis 64: 525-527. PubMed: 22116335. [PubMed] [Google Scholar]
- 13. Lai CH, Huang CK, Chen YH, Chang LL, Weng HC et al. (2009) Epidemiology of acute Q Fever, scrub typhus, and murine typhus, and identification of their clinical characteristics compared to patients with acute febrile illness in southern Taiwan. J Formos Med Assoc 108: 367-376. doi: 10.1016/S0929-6646(09)60080-2. PubMed: 19443290. [DOI] [PubMed] [Google Scholar]
- 14. Lai CH, Chen YH, Lin JN, Chang LL, Chen WF et al. (2009) Acute Q fever and scrub typhus, southern Taiwan. Emerg Infect Dis 15: 1659-1661. doi: 10.3201/eid1510.090007. PubMed: 19861068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou MY, Hung MN, Lin PS, Wang YC, Lin CC et al. (2011) Use of a single-tube nested real-time PCR assay to facilitate the early diagnosis of acute Q fever. Jpn J Infect Dis 64: 161-162. PubMed: 21519134. [PubMed] [Google Scholar]
- 16. Talkington DF, Shott S, Fallon MT, Schwartz SB, Thacker WL (2004) Analysis of eight commercial enzyme immunoassay tests for detection of antibodies to Mycoplasma pneumoniae in human serum. Clin Diagn Lab Immunol 11: 862-867. PubMed: 15358644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nir-Paz R, Michael-Gayego A, Ron M, Block C (2006) Evaluation of eight commercial tests for Mycoplasma pneumoniae antibodies in the absence of acute infection. Clin Microbiol Infect 12: 685-688. doi: 10.1111/j.1469-0691.2006.01469.x. PubMed: 16774570. [DOI] [PubMed] [Google Scholar]
- 18. Petitjean J, Vabret A, Gouarin S, Freymuth F (2002) Evaluation of four commercial immunoglobulin G (IgG)- and IgM-specific enzyme immunoassays for diagnosis of Mycoplasma pneumoniae infections. J Clin Microbiol 40: 165-171. doi: 10.1128/JCM.40.1.165-171.2002. PubMed: 11773112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beersma MF, Dirven K, van Dam AP, Templeton KE, Claas EC et al. (2005) Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the "gold standard". J Clin Microbiol 43: 2277-2285. doi: 10.1128/JCM.43.5.2277-2285.2005. PubMed: 15872256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hermann C, Graf K, Groh A, Straube E, Hartung T (2002) Comparison of eleven commercial tests for Chlamydia pneumoniae-specific immunoglobulin G in asymptomatic healthy individuals. J Clin Microbiol 40: 1603-1609. doi: 10.1128/JCM.40.5.1603-1609.2002. PubMed: 11980928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Persson K, Boman J (2000) Comparison of five serologic tests for diagnosis of acute infections by Chlamydia pneumoniae. Clin Diagn Lab Immunol 7: 739-744. PubMed: 10973447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kung CM, Wang HL (2007) Seroprevalence of Mycoplasma pneumoniae in healthy adolescents in Taiwan. Jpn J Infect Dis 60: 352-354. PubMed: 18032833. [PubMed] [Google Scholar]
- 23. Liu FC, Chen PY, Huang FL, Tsai CR, Lee CY et al. (2008) Do serological tests provide adequate rapid diagnosis of Mycoplasma pneumoniae infection? Jpn J Infect Dis 61: 397-399. PubMed: 18806352. [PubMed] [Google Scholar]
- 24. Lauderdale TL, Chang FY, Ben RJ, Yin HC, Ni YH et al. (2005) Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir Med 99: 1079-1086. doi: 10.1016/j.rmed.2005.02.026. PubMed: 16085210. [DOI] [PubMed] [Google Scholar]
- 25. Yen MY, Hu BS, Chen YS, Lee SS, Lin YS et al. (2005) A prospective etiologic study of community-acquired pneumonia in Taiwan. J Formos Med Assoc 104: 724-730. PubMed: 16385374. [PubMed] [Google Scholar]
- 26. Fernández Alvarez R, Suárez Toste I, Rubinos Cuadrado G, Torres Lana A, Gullón Blanco JA et al. (2007) Community-acquired pneumonia: aetiologic changes in a limited geographic area. An 11-year prospective study. Eur J Clin Microbiol Infect Dis 26: 495-499. doi: 10.1007/s10096-007-0323-3. PubMed: 17554569. [DOI] [PubMed] [Google Scholar]
- 27. Shibli F, Chazan B, Nitzan O, Flatau E, Edelstein H et al. (2010) Etiology of community-acquired pneumonia in hospitalized patients in northern Israel. Isr Med Assoc J 12: 477-482. PubMed: 21337816. [PubMed] [Google Scholar]
- 28. Lai CH, Chin C, Chung HC, Huang CK, Chen WF et al. (2007) Acute Q fever hepatitis in patients with and without underlying hepatitis B or C virus infection. Clin Infect Dis 45: e52-e59. doi: 10.1086/520680. PubMed: 17682980. [DOI] [PubMed] [Google Scholar]
- 29. Lai CH, Huang CK, Chin C, Chung HC, Huang WS et al. (2008) Acute Q fever: an emerging and endemic disease in southern Taiwan. Scand J Infect Dis 40: 105-110. doi: 10.1080/00365540701558722. PubMed: 17852909. [DOI] [PubMed] [Google Scholar]
- 30. Camacho MT, Outschoorn I, Tellez A, Sequí J (2005) Autoantibody profiles in the sera of patients with Q fever: characterization of antigens by immunofluorescence, immunoblot and sequence analysis. J Autoimmune Dis 2: 10. doi: 10.1186/1740-2557-2-10. PubMed: 16280092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy P, Raoult D, Razongles JJ (1989) Q-fever and autoimmunity. Eur_J Epidemiol 5: 447-453. doi: 10.1007/BF00140139. PubMed: 2691275. [DOI] [PubMed] [Google Scholar]
- 32. Cunha BA (2006) The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect 12 Suppl 3: 12-24. doi: 10.1111/j.1469-0691.2006.01393.x. PubMed: 16669925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waites KB, Talkington DF (2004) Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 17: 697-728. doi: 10.1128/CMR.17.4.697-728.2004. PubMed: 15489344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F et al. (2005) Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin Infect Dis 40: 82-88. doi: 10.1086/426440. PubMed: 15614696. [DOI] [PubMed] [Google Scholar]