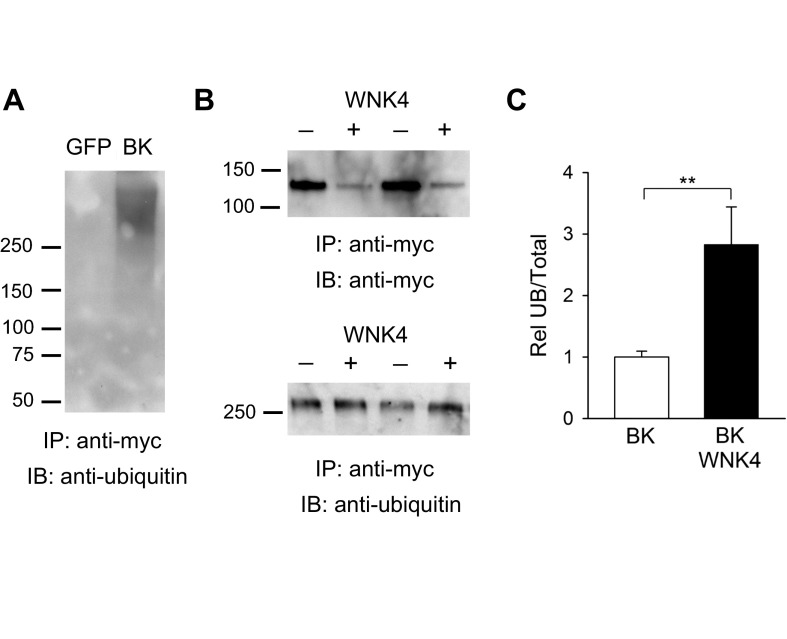
Fig. 5.
WNK4 augments BK α-subunit ubiquitination. A: the BK α-subunit is polyubiquitinated. HEK293 cells were transfected with GFP or a myc-tagged BK α-subunit. Proteins were immunoprecipitated (IP) from whole cell lysates with an anti-myc antibody. IB was performed with an anti-ubiquitin antibody to detect ubiquitinated BK α-subunit as described in materials and methods. B: WNK4 augments BK α-subunit ubiquitination. HEK293 cells were transfected with myc-tagged BK α-subunit with or without wild-type WNK4. BK α-subunit was immunoprecipitated from whole cell lysates with an anti-myc antibody. IB was performed with an anti-myc antibody to detect BK α-subunit or with an anti-ubiquitin antibody to detect ubiquitinated BK α-subunit as indicated in A. Representative blots from experiments conducted in duplicate are shown. C: summary of analyses of BK α-subunit ubiquitination. Immunoblots were quantified as described in materials and methods. The fraction of ubiquitinated BK α-subunit normalized to the total whole cell BK α-subunit expression (Rel UB/Total) is shown (n = 6, **P < 0.01, Mann-Whitney test).