Fig. 3.
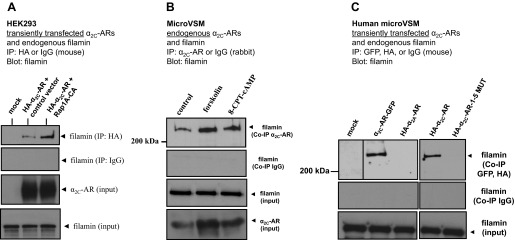
Filamin and α2C-ARs co-immunoprecipitate. A: α2C-AR-filamin interaction in transiently transfected human embryonic kidney (HEK) 293 cells. Cells were cotransfected with control vector or constitutively active Rap1A (Rap1A-CA). Coimmunoprecipitation (Co-IP) was performed with HA or control IgG (mouse) and blotted with filamin. Representative results are shown, including filamin and α2C-ARs (input). The α2C-AR-specific antibody recognizes the ∼70-kDa mature receptor form that translocates from the Golgi compartment to the cell surface. B: endogenous α2C-ARs interact with endogenous filamin in human microVSM. α2C-AR-filamin interaction under untreated (solvent control, 16 h) or treated conditions (forskolin, 10 μM, 16 h), or the selective Epac-Rap activator 8-pCPT-2′-O-Me-cAMP (8-CPT-cAMP; 100 μM, 16 h). Immunoprecipitations were performed with α2C-AR-specific antibody or control IgG (rabbit), and blotted with antibody to filamin. Representative results are shown, including filamin and α2C-ARs (input). C: α2C-AR-filamin interaction in transiently transfected human microVSM. Cells were transfected with various tagged full-length WT or mutated (1–5 MUT) α2C-ARs [green fluorescent protein (GFP) or hemagglutinin (HA)-tagged] and examined after 16 h of forskolin (10 μM) treatment. The α2A-ARs do not interact with filamin and do not Co-IP. Immunoprecipitations were performed with antibodies to the tags or control IgG (mouse) and blotted to filamin. The α2C-ARs tagged either with GFP or HA coimmunoprecipated with filamin. However, α2C-AR-1–5 MUT, like the control α2A-ARs, did not coimmunoprecipate with filamin. Expression of these constructs was confirmed by Western blotting or by imaging (not shown). Dividing line indicates noncontiguous lanes.
