Fig. 8.
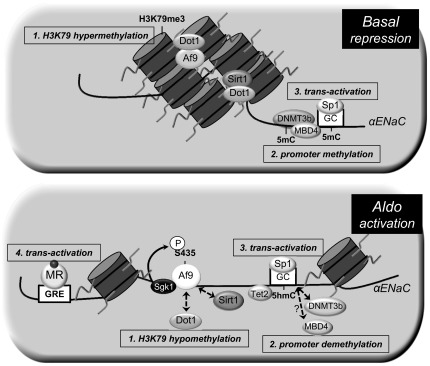
Model of the genetic and epigenetic control of basal and Aldo-stimulated transcription of the αENaC gene in mIMCD3 cells. Under basal conditions (top), the histone methyltransferase disruptor of telomeric silencing (Dot)1a is complexed with the DNA-binding protein Af9 or sirtuin (Sirt)1 in chromatin associated with specific regions of the αENaC 5′-flanking region and hypermethylates histone H3K79 (1). In addition, DNMT3b and MBD4 mediate and maintain cytosine methylation (5mC) of the αENaC promoter (2), which also constrains αENaC transcription, in part by limiting the binding and full trans-activation effect transcription factor Sp1 binding to its GC box in the αENaC promoter (3). Aldo (bottom) downregulates the expression of Dot1a, Af9, and Sirt1 (1), leading to decreased abundance of the repressor complex, and the hormone induces serum- and glucocorticoid-induced kinase 1, which phosphorylates Ser435 of Af9, causing disruption of the protein-protein interactions of Dot1a and Af9. This results in the hypomethylation of histone H3K79 and release of transcriptional repression of αENaC. In addition, DNMT3b and, presumably, MBD4 are dispersed from, and Tet2 recruited to, chromatin associated with the αENaC promoter, resulting in promoter demethylation with the conversion of 5mC to 5hmC, further de-repressing the promoter (2). The promoter demethylation maximizes Sp1 binding and trans-activation of the αENaC gene (3). Finally, the Aldo-liganded mineralocorticoid receptor (MR) binds to a glucocorticoid response element (GRE) in the αENaC promoter to further trans-activate the αENaC gene (4).
