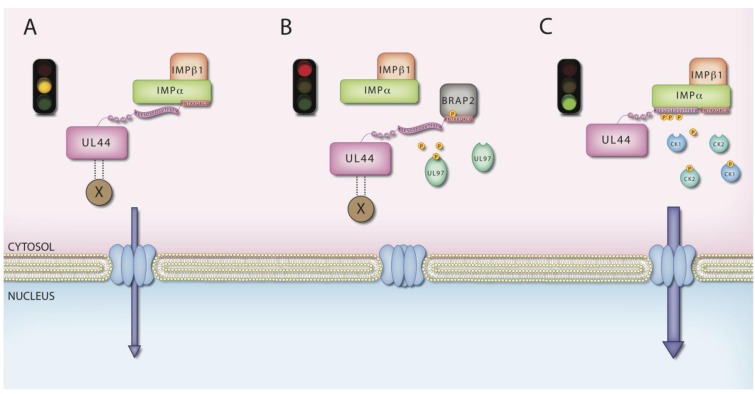
Figure 3.
Phosphorylation-regulated nuclear transport of HCMV PAP. HMCV PAP UL44 nuclear import depends on the phosphorylation of its C-terminus. (A) Unphosphorylated UL44 C-terminus can be recognized by the IMPα/β heterodimer with medium affinity. This results in a low rate nuclear import of UL44, possibly enabling interaction with other cytoplasmic factors before import occurs. X indicates either another UL44 molecule, UL54 or the viral uracil DNA glycosylase; (B) Phosphorylation of T427, as mediated by UL97 or cdk1 enables binding to the cytoplasmic retention factor BRAP2, thereby preventing IMPα/β recognition and nuclear targeting. Phosphorylation of S413, S415 and S418 by CK2 and CK1 enhances IMPα/β binding, thereby promoting rapid nuclear import, possibly preventing UL44 to interact with other partners before nuclear import (C).