Abstract
BACKGROUND: Ephrin B2 receptor (EphB2) is a target of the canonical wnt pathway implicated in colorectal carcinogenesis, and its down-regulation may be associated with adverse prognosis. We evaluated its prognostic value in resected colon cancer stratified by microsatellite status and other clinicopathologic characteristics. METHODS: We identified all cases of resected stage III colon cancer from 1995 to 2009 managed in the Capital Health district of Nova Scotia. Tissue microarrays were constructed and immunohistochemistry (IHC) for tumor EphB2 staining assigned into quartiles. Microsatellite status was evaluated by IHC for MutL homolog 1 (MLH1) and MutS homolog 2 (MSH2). Microsatellite stable tumors were defined as both MLH1/MSH2 (+/+); tumors staining otherwise were classified with microsatellite instability (MSI-H). Primary and secondary outcomes were disease-free survival (DFS) and overall survival (OS), respectively. RESULTS: We identified 159 cases with sufficient tissue for microarray analysis having a median follow-up of 3.47 years (range, 0.14–14). Median age was 61, 52% were male, 40% had an event, and 29% died. MSI-H was present in 18 (13%). Univariate analysis of EphB2 expression on DFS and OS showed a hazard ratio (HR) of 2.00 (P = .01) and 2.14 (P = .03), respectively. Multivariate analysis of EphB2 expression on DFS and OS showed an HR of 2.24 and 2.23, respectively, with tumor IHC ≤ 50%. CONCLUSIONS: In this cohort, decreased EphB2 expression was an independent prognostic factor for recurrence and death and may have prognostic relevance in tumors with MSI-H. However, this would require prospective validation in a larger study.
Introduction
Treatment advances for colorectal cancer have changed significantly over the last decade in both early-stage and metastatic settings. However, colorectal cancer remains one of the leading causes of mortality, with rectal cancer having a worse stage-specific prognosis than colon cancer. Despite the development and adoption of targeted systemic therapies for metastatic disease, the median survival remains less than 2 years, consumes significant healthcare resources, and is associated with rising treatment costs [1–5]. A patient selection strategy that is currently lacking is one that may further guide systemic therapy by stratifying patients based on their tumor biology as well as stage-specific prognosis. This may allow foregoing adjuvant chemotherapy in some patients with a sufficiently good prognosis or, conversely, treating those with higher stage-specific risk.
Significant progress has been made in solid tumor oncology with the identification of biologically distinct cancer-specific subsets. The application of this knowledge to treatment decision-making has led to clinically improved patient outcomes in metastatic colorectal cancer; testing specific k-RAS mutations for epidermal growth factor receptor inhibitor therapy is one example [6,7]. Furthermore, colon cancer has two dominant models of carcinogenesis, the microsatellite instability pathway and chromosomal instability, almost exclusive of each other [8,9]. Testing tumors for expression of the mismatch repair proteins MutL homolog 1 (MLH1), MutS homolog 2 (MSH2), MutS homolog 6 (MSH6), and postmeiotic segregation increased 2 (PMS2) by immunohistochemistry (IHC) is a practical way to assess microsatellite instability. In lymph node-positive colon cancer, observational studies and retrospective analyses of randomized clinical trials demonstrate an improved prognosis for tumors with high levels of microsatellite instability (MSI-H) and reduced benefit from adjuvant chemotherapy [10,11].
Ephrins were initially identified when screening for a tyrosine kinase domain of the viral oncogene v-fps [12]. Their roles are diverse but are largely related to maintaining homeostasis of the cellular environment, particularly in cellular orientation, motility, and microvasculature [13–17]. The diverse roles of ephrin signaling in the regulation of cell migration and tissue assembly have led to their study in multiple fields, including the pathogenesis of solid tumors. The ephrin B2 receptor (EphB2) is a subtype of the ephrin receptor family, the largest receptor tyrosine kinase family of transmembrane proteins. EphB2 is a multifunctional tyrosine kinase receptor that has showed prognostic significance in various tumor types, including colorectal cancer [15,18–20]. The extracellular domain is capable of recognizing signals from the cells' environment and influencing cell-cell interaction and cell migration. Preclinical evidence and observational studies have shown that progressive loss of functional EphB2 may be associated with a worse prognosis in multiple stages of colorectal cancer [18,19,21]. EphB2 is also believed to be a tumorigenic marker in colorectal cancer [22]. However, the largest clinicopathologic correlative study on EphB2 as a prognostic factor for colorectal cancer had evaluated patient cohorts with both colon and rectal cases analyzed together, limited information on systemic therapy, and mixed disease stages [23]. We conducted a single-center retrospective study of the prognostic value of EphB2 in patients with resected lymph node- positive colon cancer. Common clinical and pathology data were collected, and individual patients' microsatellite status was assessed on the basis of the premise of differing carcinogenic pathways.
Methods
Patients and Data Collection
We identified all cases of Union for International Cancer Control stage III colon cancer at the Capital District Health Authority (CDHA) from January 1995 to October 2009. All relevant clinical and pathology data were collected and abstracted from patients' medical records. For study inclusion, patients must have received ≥67% total planned dose of 6 months adjuvant chemotherapy. Cases without positive lymph nodes, or those with mesenteric or small omental tumor deposits without lymph node involvement (i.e., nodal stage N1c), were excluded. No patients received neoadjuvant chemotherapy. Pathology specimens were reviewed centrally at the Queen Elizabeth II Health Sciences Centre, and two pathology reviewers (T.A. and W.Y.H.) were blinded to outcomes. On the basis of these criteria, 159 cases had sufficient tissue for microarray analysis and constitute our target cohort. This study was approved by the CDHA Research Ethics Board (File No. CDHA-RS/2011-048).
Tissue Microarray and IHC Analysis
Original hematoxylin and eosin slides from all cases were reviewed to confirm the diagnosis of colon adenocarcinoma. Tissue micro-arrays including triplicate 0.5-mm or duplicate 2-mm punches were constructed from formalin-fixed tissue in archived paraffin wax blocks. The 0.5-mm punches were collected using the Manual Tissue Arrayer MTA-1 (Beecher Instruments, Sun Prairie, WI), and the 2-mm punches were collected using the Tissue-Tek Quick-Ray Microarray System (Sakura Finetek, Torrance, CA). IHC stains for MLH1 (G168-15; BD Pharmingen, Franklin Lakes, NJ), MSH2 (FE11; BD Pharmingen), MSH6 BC/44 (Biocare Medical, Concord, CA), and PMS2 EPR3947 (Cell Marque, Rocklin, CA) were applied to 5-µm sections according to our previously published protocol [24].
IHC staining for EphB2 was performed manually as follows. After dewaxing and rehydration, sections were pretreated with peroxidase blocking buffer (120 mM Na2HPO4, 43 mM citric acid, 30 mM NaN3, 0.2% H2O2; pH 5.8) for 15 minutes. Antigen retrieval was performed by autoclaving sections for 20 minutes at 121°C and 1 atm in 20 mM sodium citrate buffer (pH 6.0). Slides were washed three times in phosphate-buffered saline (PBS) before blocking for 20 minutes with 0.05% BSA Fraction V (Roche, Indianapolis, IN). Slides were washed three times in PBS and incubated with goat anti-EphB2 (AF467; R&D Systems, Minneapolis, MN) at 1:200 dilution in 0.05% BSA at 4°C. Sections were washed three times in PBS and incubated with rabbit anti-goat IgG (Jackson ImmunoResearch, West Grove, PA) for 1 hour at room temperature at 1:5000 dilution in 0.05% BSA, followed by incubation with Powervision HRP anti-rabbit IgG (Novocastra/Leica Microsystems, Wetzlar, Germany) for 45 minutes at room temperature. Slides were washed in PBS, developed with DAB, and counterstained with hematoxylin.
IHC stains were interpreted by consensus of two study authors (T.A. and W.Y.H.). The stains were interpreted independently by each reviewer, and in cases where there was disagreement on independent review, a consensus was reached by a secondary review of both pathologists together using a multiheaded microscope. MLH1, MSH2, MSH6, and PMS2 expression was considered intact when any proportion of the tumor cells showed nuclear staining. Loss of expression of MLH1, MSH2, MSH6, and PMS2 was defined as complete loss of nuclear expression in the tumor cells when internal control lymphocytes and stromal cells had appropriate nuclear immunopositivity. EphB2 staining was interpreted on the basis of the proportion of cells with complete membrane staining of any intensity. The proportion of tumor cells with EphB2 immunopositivity was determined by visual estimation at x200 magnification. The proportion of immunopositive cells for each case was recorded in one of four quartiles: 0% to 25%, 26% to 50%, 51% to 75%, or 76% to 100% positive tumor cells. Benign colonic mucosal cells at the base of the crypts with complete membrane staining served as internal positive controls for EphB2, while surface epithelial cells served as internal negative controls. In tumors with loss of any mismatch repair protein by IHC, DNA analysis for microsatellite instability was performed according to our previously published protocol [24]. We interpreted tumors with instability at greater than or equal to two mononucleotide loci (≥40%) as having a high rate of microsatellite instability (MSI-H). Cases with instability at a single locus (20%) were classified as low probability of MSI-H. Cases without instability at any locus were categorized as microsatellite stable (MSS).
Statistical Considerations
Time-to-event analysis, either last known follow-up, first documented cancer recurrence, or death, was performed by the Kaplan-Meier method. Disease-free survival (DFS) was defined as the time from surgical tumor resection to recurrence or death, and overall survival (OS) was defined as the time from surgery to death. The log-rank test was performed to compare survival outcomes in groups stratified by EphB2 and microsatellite status.
The correlation between clinicopathologic characteristics and DFS or OS was examined using a univariate Cox proportional hazards model and tested for significance (Table 2). Proportional hazards assumption for each variable was tested by using the supremum test, graphical assessment using the log-negative-log survival curves to test for parallelism and plots of the Schoenfeld residuals. Continuous variables were tested for linearity on the log hazard scale.
Table 2.
Univariate and Multivariate Analyses.
| Univariate Analysis | |||||||||
| DFS | OS | ||||||||
| HR [95% Confidence Interval (CI)] | P Value | HR (95% CI) | P Value | ||||||
| Age (< vs ≥ median) | 0.796 (0.477, 1.328) | .3824 | 0.553 (0.301, 1.014) | .0557* | |||||
| Gender (female vs male) | 0.645 (0.384, 1.084) | .0980* | 0.585 (0.317, 1.080) | .0865* | |||||
| T stage (T3/4 vs T1/2) | 2.049 (0.880,4.785) | .0961* | 1.751 (0.689, 1.751) | .2388 | |||||
| N stage (N2 vs N1) | 2.128 (1.258, 3.597) | .0049* | 2.114 (1.144, 3.891) | .0168* | |||||
| Grade (III vs I/II) | 1.896 (1.097, 3.277) | .0219* | 1.955 (1.041, 3.671) | .0371* | |||||
| Histology (adenocarcinoma vs mucinous adenocarcinoma) | 0.729 (0.331, 1.608) | .4340 | 0.725 (0.285, 1.844) | .4991 | |||||
| Location | |||||||||
| Left vs transverse | 0.376 (0.168, 0.843) | .0492* | 0.397 (0.156, 1.007) | .1410 | |||||
| Right vs transverse | 0.412 (0.187,0.906) | - | 0.451 (0.181, 1.124) | - | |||||
| Chemotherapy | |||||||||
| 5-FU/raltitrexed vs capecitabine | 1.372 (0.425, 4.431) | .2709 | 1.633 (0.224,12.354) | - | |||||
| FOLFOX vs capecitabine | 0.286 (0.030, 2.755) | - | 0 (0, NA)† | .8836 | |||||
| Microsatellite status (MSI-H vs MSS) | 1.440 (0.574, 3.611) | .4368 | 5.067 (0.697, 36.826) | .1088* | |||||
| EphB2 (0–50% vs 51–100%) | 2.000 (1.149, 3.484) | .0143* | 2.146 (1.081, 4.261) | .0291* | |||||
| Multivariate Analysis | |||||||||
| DFS | OS | ||||||||
| HR‡ | 95% CI | P Value | HR‡ | 95% CI | P Value | ||||
| Age (median = 61) | - | - | - | ||||||
| > vs ≤ Median | 2.41 | 1.32–4.40 | .004 | ||||||
| N stage | |||||||||
| N2 vs N1 | 2.59 | 1.51–4.44 | < .001 | 2.56 | 1.41–4.66 | .002 | |||
| Location | |||||||||
| Left vs transverse | 0.357 | 0.158–0.811 | .014 | ||||||
| Right vs transverse | 0.373 | 0.166–0.837 | .017 | ||||||
| EphB2 Expression | |||||||||
| 0–50% vs 51–100% | 2.24 | 1.27–3.93 | .005 | 2.23 | 1.18–4.27 | .014 | |||
Variables significant at P < .2 were considered for multivariate analysis.
Upper bound of CI cannot be estimated because there are no events in the FOLFOX arm.
Reported HRs are for outcome events.
Only variables that met the proportionality assumption and were significant at P < .2 were entered into the multivariate model (Table 2). Variables entered into the final multivariate model were chosen using stepwise selection. The final model was then tested for overall goodness-of-fit test [25].
Results
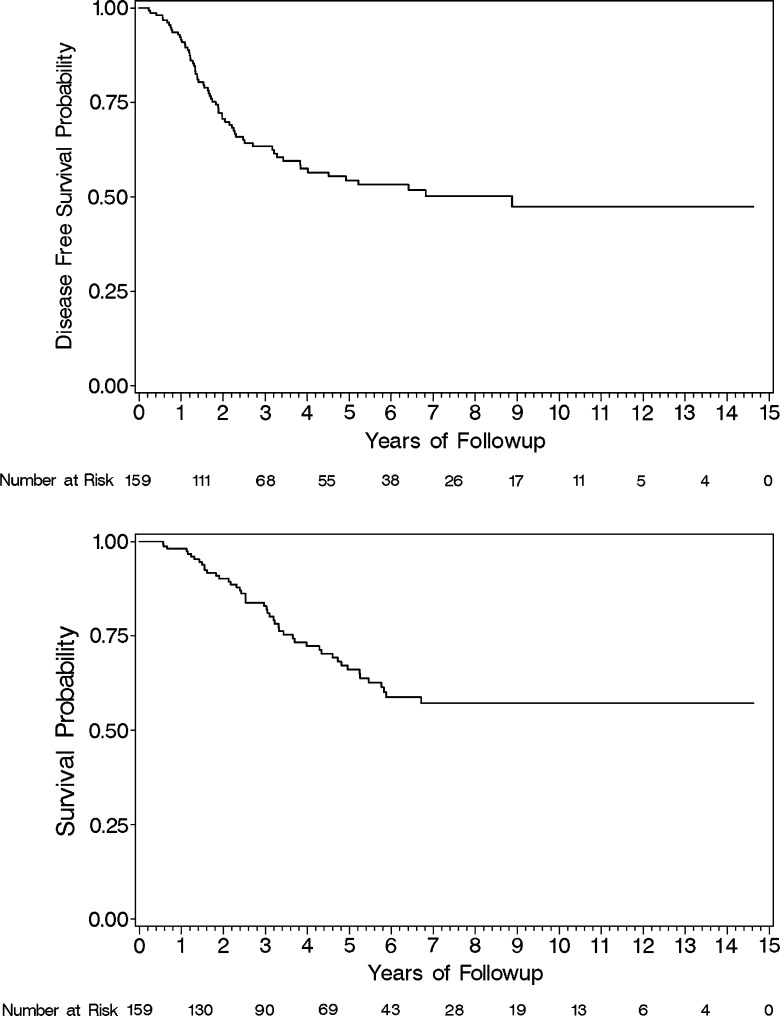
Patient demographics, EphB2, and microsatellite data were summarized using descriptive statistics performed with SAS software version 9.2. The clinical and pathologic characteristics of the target cohort are shown in Table 1A, and common clinicopathologic associations with EphB2 are summarized in Table 1B. Eighteen of 159 tumors showed loss of expression of one or more of the mismatch repair proteins MLH1, MSH2, MSH6, and PMS2 by IHC. An example of loss of mismatch repair proteins is shown in Figure 1. The tumors with loss of mismatch repair proteins were all tested for microsatellite instability and confirmed to be MSI-H. The tumor tissue specimens demonstrated marked variation in the proportion of cells with complete membrane staining for EphB2, ranging from complete absence of staining in all tumor cells to uniform immunopositivity for EphB2 in essentially all tumor cells (Figure 2). The median age was 61.5 and distributed almost equally between genders. Nearly 40% of patients had a recurrence, with the proportion of isolated metastases only to the liver or lung at 30% and 13%, respectively. The majority of cases were typical adenocarcinoma and had invasion at least into the muscle layer, with most having N1 disease. Almost two thirds were scored as having an intermediate tumor grade. Eighty percent received either adjuvant 5-fluorouracil (5-FU) or raltitrexed chemotherapy. DFS and OS for the study cohort are shown in Figure 3.
Table 1A.
Patient Characteristics and EphB2 Clinicopathologic Factors.
| N | Percentage | |
| Gender | ||
| Male | 82 | 52 |
| Female | 77 | 48 |
| T stage | ||
| T1 or T2 | 24 | 15 |
| T3 or T4 | 135 | 85 |
| N stage | ||
| N1 | 112 | 70 |
| N2 | 46 | 29 |
| Missing | 1 | 1 |
| Tumor grade | ||
| I—Well | 16 | 10 |
| II—Moderate | 102 | 64 |
| III—Poor | 36 | 22 |
| Missing | 5 | 3 |
| Histologic subtype | ||
| Adenocarcinoma | 135 | 85 |
| Mucinous | 21 | 13 |
| Signet ring carcinoma | 2 | 1 |
| Missing | 1 | 1 |
| Chemotherapy regimen | ||
| 5-FU or raltitrexed | 127 | 80 |
| Capecitabine | 16 | 10 |
| FOLFOX | 16 | 10 |
| Location | ||
| Left | 72 | 45 |
| Right | 72 | 45 |
| Transverse | 12 | 8 |
| Missing | 3 | 2 |
| Surgical margins | ||
| Negative | 146 | 92 |
| Positive | 7 | 4 |
| Missing | 6 | 4 |
| Proportion of EphB2 tumor staining (%) | ||
| 0–25 | 65 | 40 |
| 26–50 | 19 | 12 |
| 51–75 | 35 | 22 |
| 76–100 | 40 | 25 |
| Microsatellite status | ||
| Stable (MSS) | 141 | 89 |
| Unstable (MSI-H) | 18 | 11 |
Table 1B.
EphB2 Associations with Clinicopathologic Factors.
| EphB2 Expression | P Value | ||||
| Low (0–50%) | High (51–100%) | ||||
| N | Percentage | N | Percentage | ||
| Cases | 84 | 52 | 75 | 48 | |
| Age | .62* | ||||
| Median | 61.34 | - | 61.49 | - | |
| Gender | .92 | ||||
| Male | 84 | 51.19 | 52 | 39 | |
| Female | 41 | 48.81 | 48 | 36 | |
| T stage | .46 | ||||
| T1 or T2 | 11 | 13.1 | 13 | 17.33 | |
| T3 or T4 | 73 | 86.9 | 62 | 82.67 | |
| N stage | .45 | ||||
| N1 | 61 | 73.49 | 51 | 68 | |
| N2 | 22 | 26.51 | 24 | 32 | |
| Tumor grade | .08 | ||||
| Grade I or II | 59 | 71.08 | 59 | 83.1 | |
| Grade III | 24 | 28.92 | 12 | 16.9 | |
| Histologic subtype | .63 | ||||
| Adenocarcinoma NOS | 72 | 86.75 | 63 | 84 | |
| Mucinous Location | 10 | 12.05 | 11 | 14.67 | .15† |
| Left | 35 | 42.68 | 37 | 50 | |
| Right | 37 | 45.12 | 35 | 47.3 | |
| Transverse | 12 | 10 | 2 | 2.7 | |
| Microsatellite status | .45 | ||||
| Stable (MSS) | 73 | 86.9 | 68 | 90.7 | |
| Unstable (MSI-H) | 11 | 13.1 | 7 | 9.3 | |
t test performed.
Global P value from logistic regression.
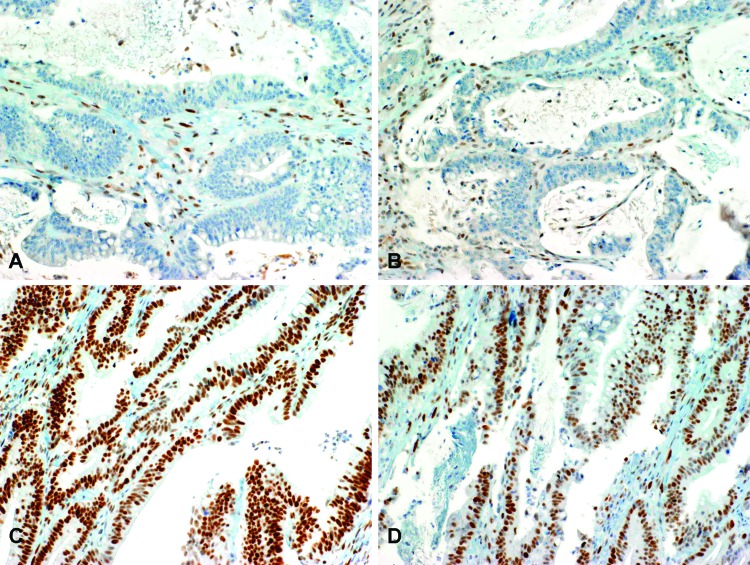
Figure 1.
Mismatch repair protein IHC. (A) IHC staining shows absence of nuclear expression of MLH1 in malignant glands, while internal control lymphocytes and stromal cells have intact MLH1 immunopositivity. (B) The same tumor displays loss of PMS2 in tumor cell nuclei. (C) IHC staining for MSH2 in the same tumor shows intact nuclear expression. (D) Intact MSH6 in the same case. Original magnification, x200 (A–D).
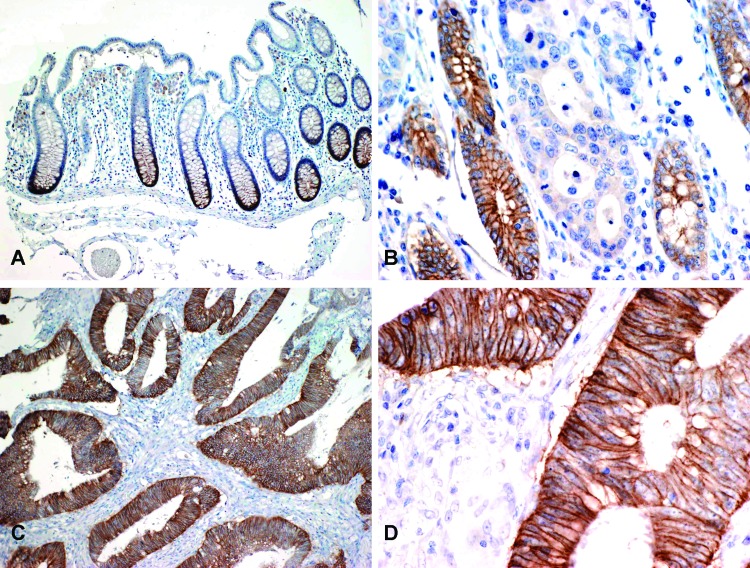
Figure 2.
EphB2 IHC. (A) IHC staining for EphB2 in normal colonic mucosa shows complete membrane staining at the base of the crypts, which is lost toward the mucosal surface (original magnification, x100). (B) Adenocarcinoma with loss of EphB2 expression infiltrates between several mucosal crypts that retain strong EphB2 expression and serve as an internal positive control (original magnification, x200). (C) A different tumor with diffuse EphB2 expression (original magnification, x100). (D) The same tumor at higher magnification highlights the complete membranous EphB2 staining pattern (original magnification, x400).
Figure 3.
Target cohort DFS (top) and OS (bottom).
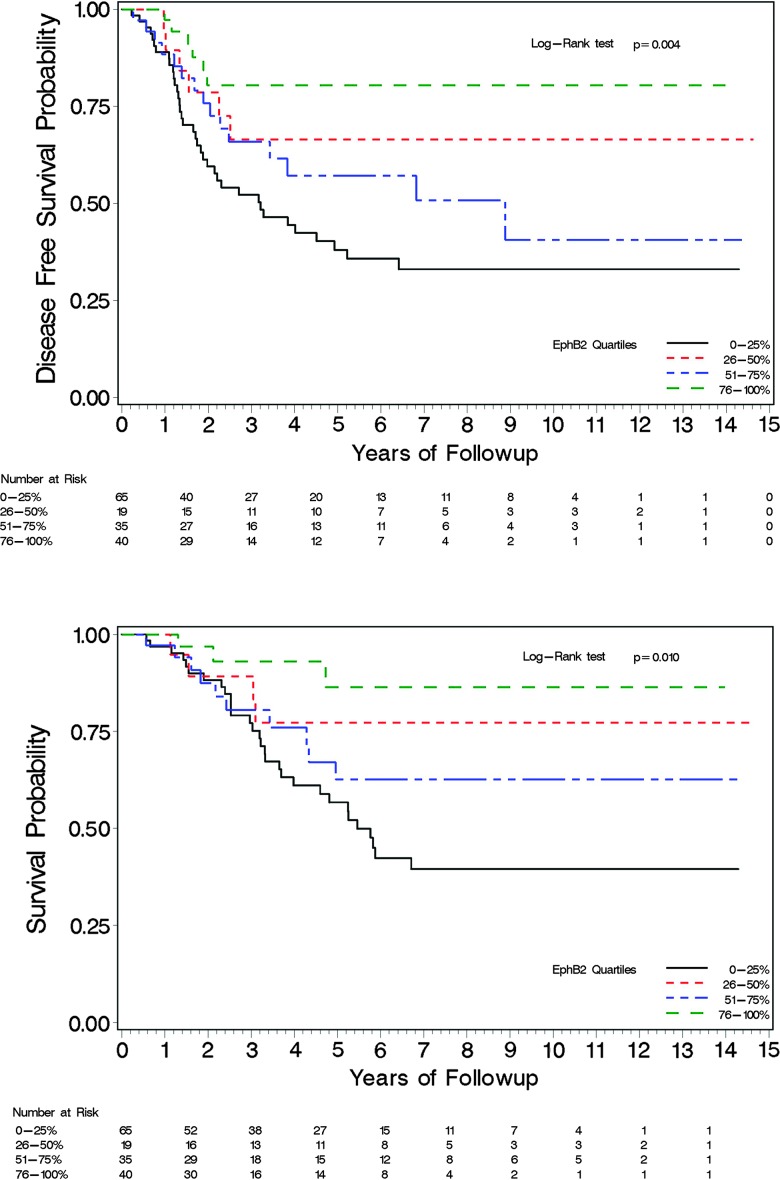
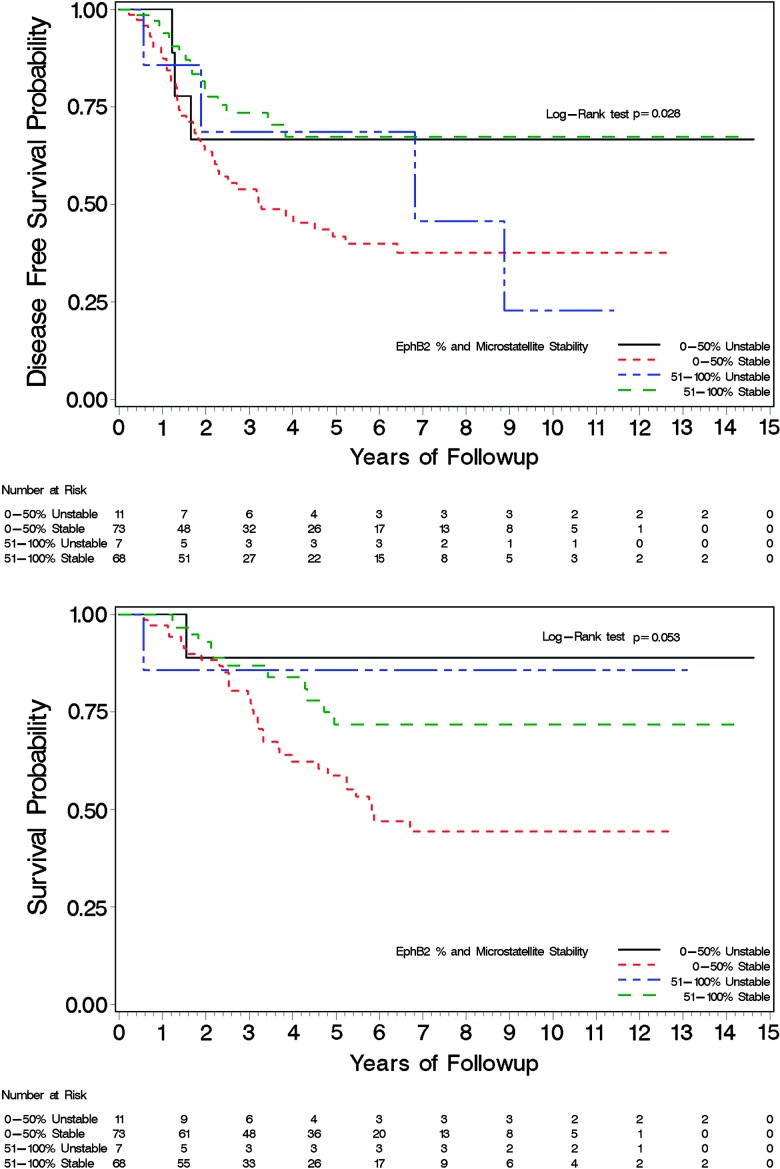
Our data show a statistically significant improvement in DFS and OS in patients whose tumors expressed EphB2 and worse survival outcomes with lower expression (Figures 4 and 5). EphB2 cases with ≤50% of tumor IHC staining represented 52% of the study cohort. The data demonstrate a correlation with 5-year DFS of 80%, 67%, 57%, and 38% and 5-year OS of 86%, 63%, 77%, and 57% corresponding to tumor EphB2 IHC in categorical quartiles of 76% to 100%, 51% to 75%, 26% to 50%, and 0% to 25%, respectively. Relative to other quartiles, there are fewer subjects in the 26% to 50% quartile grouping (n = 19), and only four died in this group and six died or recurred. The actual frequency of mortality is higher in this group than seen in the 51% to 75% quartile, where 10 of 35 subjects died and 15 of 35 subjects had a death or reoccurrence event. DFS and OS outcomes stratified by both EphB2 and micro-satellite status are shown in Figure 5, which demonstrate a statistically significant improved survival in those with >50% EphB2 expression in the MSS subset. Observed OS and DFS in MSI-H tumors were better than those having MSS tumors; however, these outcomes are not statistically significant as the subset of patients with MSI-H comprised 18 patients, and after 5 years of follow-up, there are very few events and patients at risk. In a separate exploratory analysis, MSI-H was associated with tumor location and grade, with 72% of these tumors being right sided and 60% high grade. The association of EphB2 and grade as well as to tumor location is also suggested in Table 1B; however, they do not reach the level of statistical significance. Analysis of IHC for PMS2 showed no discordance in any cases of intact expression of MLH1 (data not shown).
Figure 4.
DFS (top) and OS (bottom) stratified by EphB2 quartiles.
Figure 5.
DFS (top) and OS (bottom) stratified by EphB2 and microsatellite status.
Univariate and multivariate analyses for DFS and OS are summarized in Table 2. Except for age and microsatellite status, their association on a number of factors correlated with DFS and OS, including EphB2 expression. Multivariate analyses using the stepwise model for DFS and OS show that EphB2 down-regulation, at a median threshold of 50% immunostaining, is an independent prognostic factor for recurrence and death with similar proportional hazards for each outcome. Higher nodal stage was the only other variable to demonstrate an independently worse prognosis in multivariate analysis for both DFS and OS.
Discussion
In this retrospective study, we evaluated the prognostic significance of EphB2 in a uniform cohort of stage III treated colon cancer patients. This was done to minimize potential confounding factors, make the target cohort more clinically homogeneous, and facilitate collection of recurrence data. After accounting for common clinical and pathologic factors, both univariate and multivariate analyses on DFS and OS demonstrate that down-regulation of EphB2 is an independent prognostic factor for recurrence and death (Table 2). In multivariate analyses, the nodal stage was the only other independent prognostic factor associated with both outcome end points (Table 2). Age was not retained in the multivariate model as an independent prognostic factor for DFS but was retained for OS, which is clinically logical. Older individuals are more likely to die than younger ones independent of other factors; however, age alone would not be expected to have an independent association on recurrence. Tumor location was observed to be independently associated with DFS but not OS in multivariate analyses, and our speculation is that this may be linked to tumor microsatellite status. If true, our sample size of patients having MSI-H tumors is too small and insufficiently powered to show a statistically meaningful result (Table 1B and Figure 5). For instance, MSI-H tumors with >50% EphB2 expression seemed to have a worse DFS but a favorable OS. However, after 5 years of median follow-up, the number at risk in this patient subset is only three patients, and interventions such as conversion surgery of isolated metastases or the occurrence of a single event would have significant changes to the survival curves.
A number of other studies have examined the prognostic significance of EphB2 in colorectal cancer. Although this is a single-center study, our target cohort's characteristics appear similar to those in other Canadian cancer registries [26]. Our data are comparable to results of a very similar study that showed progressive loss of EphB2 was associated with higher tumor stage and grade, with similar IHC and scoring system methods to ours (i.e., based on proportion of cells staining and not intensity of staining). As with our study, this suggests that loss of function of EphB2 is associated with cancer progression and consequently an adverse prognosis [21]. Another study that evaluated normal colorectal epithelium, adenoma, primary adenocarcinoma, and metastases using IHC showed that decreased expression of EphB2 was associated with a shorter median survival [23]. This was performed in a cohort of mixed colon and rectal cancers, limited data on systemic treatment, and mixed stages, which may be confounding factors to survival outcomes. Most recently, results from a study that investigated EphB2 and its associated signaling suggested EphB2 as a potential colonic stem cell marker [22].
The membranous staining pattern of the EphB2 antibody used in this study was relatively straightforward to interpret by light microscopy. However, results similar to another study [21] may be difficult to reproduce using other clones of an EphB2 antibody. Our group has previously worked with a different EphB2 antibody clone (rabbit polyclonal anti-EphB2; Abnova, Taipei, Taiwan). This assay produced a cytoplasmic staining pattern that typically diffusely stained all tumor cells, with only differences in the intensity of staining noted between tumors. Assessment of differences in staining intensity was difficult, and we had greater concerns about reproducibility of the results using this clone. The intensity of EphB2 staining with this polyclonal antibody was not associated with a significant difference in DFS or OS [27].
While the EphB2 antibody used in the present study has prognostic significance and is less challenging to assess for pathologists, several issues remain before adoption of this IHC stain as a routine prognostic marker. Further study is required to formally assess the intraobserver and interobserver variability in interpretation of the stain. The present study also used tissue microarrays, which include a very small (0.5–2 mm) sample of the tumor rather than whole sections. Previous studies of other IHC stains, such as Her2/neu that similarly has a membranous staining pattern to EphB2 and is also quantified according to proportion of positive tumor cells, have shown greater than 95% correlation between tissue microarray and whole tissue sections [28]. In spite of the strong performance of tissue microarrays in other studies, a prospective study of EphB2 IHC using whole tissue sections for IHC is necessary to validate EphB2 as a prognostic marker for use in routine practice.
We recognize some inherent and potential limitations in this study. Because the entire cohort was treated with adjuvant chemotherapy, it is not possible to evaluate the outcomes of treatment of naïve patients. However, adjuvant chemotherapy has long been the standard of care for stage III colon cancer, and it would not be feasible to study this population otherwise as the sample size would be too small. We confined our cohort to patients in the Halifax Regional Municipality or those treated in CDHA to facilitate optimal collection of recurrence data because disease-specific incidence and mortality data are collected by Cancer Care Nova Scotia and the Nova Scotia Provincial Archives, respectively. A potential disadvantage to geographically constraining our target cohort may contribute to selection and referral bias.
Conclusions
Ephrin receptors are important transmembrane signaling proteins, and EphB2 specifically seems to have a significant role in colorectal epithelial cell localization and motility, as well as the stromal micro-environment, particularly the vasculature. Down-regulation of these key functions may account for the observed decrease in survival outcomes in colorectal cancer, reported in our and other similar studies, by augmenting invasion and metastasis, and may represent its potential role as a stem cell maker in colorectal cancer. Further studies with a larger sample size will be required to examine the potential impact of MSI-H, particularly because microsatellite instability is believed to contribute to a different mechanism of carcinogenesis. On the basis of our results, EphB2 appears to provide relevant prognostic discrimination in lymph node-positive colon cancer independent of standard clinical and pathologic factors.
Acknowledgments
The authors thank Dr Eduard Batlle of Institució Catalana de Recerca i Estudis Avançats (ICREA, Barcelona, Spain) for his technical supports of EphB2 IHC and critical comments of manuscript.
Footnotes
This study was funded by the Capital Health Category 3 Competitive Research grant and the Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Sciences Centre, both in Halifax, Nova Scotia. None of the authors have any financial or other conflicts of interest to disclose.
References
- 1.Asseburg C, Frank M, Köhne CH, Hartmann JT, Griebsch I, Mohr A, Osowski U, Schulten J, Mittendorf T. Cost-effectiveness of targeted therapy with cetuximab in patients with K-ras wild-type colorectal cancer presenting with initially unresectable metastases limited to the liver in a German setting. Clin Ther. 2011;33:482–497. doi: 10.1016/j.clinthera.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 3.Hsu TC, Chen HH, Yang MC, Wang HM, Chuang JH, Jao SW, Chiang HC, Wen CY, Tseng JH, Chen LT. Pharmacoeconomic analysis of capecitabine versus 5-fluorouracil/leucovorin as adjuvant therapy for stage III colon cancer in Taiwan. Value Health. 2011;14:647–651. doi: 10.1016/j.jval.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Rinaldi F, George E, Adler AI. NICE guidance on cetuximab, bevacizumab, and panitumumab for treatment of metastatic colorectal cancer after first-line chemotherapy. Lancet Oncol. 2012;13:233–234. doi: 10.1016/s1470-2045(12)70044-x. [DOI] [PubMed] [Google Scholar]
- 5.Shiroiwa T, Takeuchi T, Fukuda T, Shimozuma K, Ohashi Y. Cost-effectiveness of adjuvant FOLFOX therapy for stage III colon cancer in Japan based on the MOSAIC trial. Value Health. 2012;15:255–260. doi: 10.1016/j.jval.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 7.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 8.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 9.Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol. 2009;4:343–364. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]
- 10.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, Kim GP, Yothers G, Allegra C, Moore MJ, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- 13.Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 15.Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- 16.Rüegg C, Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol Life Sci. 2003;60:1135–1157. doi: 10.1007/s00018-003-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrinB2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 18.Alazzouzi H, Davalos V, Kokko A, Domingo E, Woerner SM, Wilson AJ, Konrad L, Laiho P, Espín E, Armengol M, et al. Mechanisms of inactivation of the receptor tyrosine kinase EPHB2 in colorectal tumors. Cancer Res. 2005;65:10170–10173. doi: 10.1158/0008-5472.CAN-05-2580. [DOI] [PubMed] [Google Scholar]
- 19.Lugli A, Spichtin H, Maurer R, Mirlacher M, Kiefer J, Huusko P, Azorsa D, Terracciano L, Sauter G, Kallioniemi OP, et al. EphB2 expression across 138 human tumor types in a tissue microarray: high levels of expression in gastrointestinal cancers. Clin Cancer Res. 2005;11:6450–6458. doi: 10.1158/1078-0432.CCR-04-2458. [DOI] [PubMed] [Google Scholar]
- 20.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15:419–433. doi: 10.1016/j.cytogfr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 22.Merlos-Suárez A, Barriga FM, Jung P, Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Muñoz P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Jubb AM, Zhong F, Bheddah S, Grabsch HI, Frantz GD, Mueller W, Kavi V, Quirke P, Polakis P, Koeppen H. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res. 2005;11:5181–5187. doi: 10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- 24.Arnason T, Sapp HL, Rayson D, Barnes PJ, Drewniak M, Nassar BA, Huang WY. Loss of expression of DNA mismatch repair proteins is rare in pancreatic and small intestinal neuroendocrine tumors. Arch Pathol Lab Med. 2011;135:1539–1544. doi: 10.5858/arpa.2010-0560-OA. [DOI] [PubMed] [Google Scholar]
- 25.Grønnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 26.Canadian Cancer Society's Steering Committee on Cancer Statistics, author. Canadian Cancer Statistics 2012. Toronto, Ontario: Canadian Cancer Society; 2012. http://www.cancer.ca/∼/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2012-English.pdf. [Google Scholar]
- 27.Drucker A, Arnason T, Yan S, Thompson K, Huang W. Prognostic significance of ephrin B2 and microsatellite status in stage III colon cancer. Ann Oncol. 2010;21(Supplement 6) Abstract P-0179. [Google Scholar]
- 28.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]