Significance
Classic embryological studies suggested that small groups of cells called organizers change the fate of neighboring tissues through inductive processes. In these studies it was also postulated that the receiving tissue has to be “competent” to respond to the inducer in a specific way. In the posterior forebrain, the signaling factor Sonic hedgehog is released from an organizer called the zona limitans intrathalamica and induces GABAergic and glutamatergic neurons of the thalamus. Here we demonstrate that the differential expression of two transcription factors, Irx3 and Pax6, spatially limits the area of competence for these inductions.
Keywords: cell signaling, neural development
Abstract
During embryonic development, the presumptive GABAergic rostral thalamus (rTh) and glutamatergic caudal thalamus (cTh) are induced by Sonic hedgehog (Shh) signaling from the zona limitans intrathalamica (ZLI) at the rostral border of the thalamic primordium. We found that these inductions are limited to the neuroepithelium between the ZLI and the forebrain–midbrain boundary, suggesting a prepattern that limits thalamic competence. We hypothesized that this prepattern is established by the overlapping expression of two transcription factors: Iroquois-related homeobox gene 3 (Irx3) posterior to the ZLI, and paired box gene 6 (Pax6) anterior to the forebrain–midbrain boundary. Consistent with this assumption, we show that misexpression of Irx3 in the prethalamus or telencephalon results in ectopic induction of thalamic markers in response to Shh, that it functions as a transcriptional repressor in this context, and that antagonizing its function in the diencephalon attenuates thalamic specification. Similarly, misexpression of Pax6 in the midbrain together with Shh pathway activation results in ectopic induction of cTh markers in clusters of cells that fail to integrate into tectal layers and of atypical long-range projections, whereas antagonizing Pax6 function in the thalamus disrupts cTh formation. However, rTh markers are negatively regulated by Pax6, which itself is down-regulated by Shh from the ZLI in this area. Our results demonstrate that the combinatorial expression of Irx3 and Pax6 endows cells with the competence for cTh formation, whereas Shh-mediated down-regulation of Pax6 is required for rTh formation. Thus, thalamus induction and patterning depends both on a prepattern of Irx3 and Pax6 expression that establishes differential cellular competence and on Shh signaling from the ZLI organizer.
It was recognized more than a century ago that inductive processes during embryogenesis depend not only on the inducing agent but also on the ability of the receiving cells to respond to this inducer, called “cellular competence” (1). During vertebrate brain development the neuroepithelium is subdivided into distinct areas that give rise to different neuronal subtypes. This process is regulated by signaling factors that are released from local signaling centers or “organizers” (2). The molecular nature of these organizer signals is relatively well understood; however, comparably little is known about how areas of differential cellular competence are established in the neural tube.
In the posterior forebrain (diencephalon) a local signaling center, the zona limitans intrathalamica (ZLI), releases Sonic hedgehog (Shh) that is responsible for patterning the prethalamus and thalamus. In the latter, high levels of Shh induce the rostral thalamus (rTh) that gives rise to GABA-producing (GABAergic) neurons immediately adjacent to the ZLI, whereas lower levels induce the glutamatergic caudal thalamus (cTh) at some distance (3–8). Formation of the rTh also depends on Shh from the diencephalic basal plate (9). These inductions only occur posterior to the ZLI, whereas other cell types are induced by Shh elsewhere in the neural tube, suggesting the existence of a prepattern that determines where cells are competent to form thalamic neurons in response to Shh. The homeodomain transcription factor Irx3 has previously been suggested to limit thalamic competence to the neuroepithelium posterior to the ZLI: ectopic expression of Irx3 anterior to the ZLI resulted in a mirrored duplication of the rTh and cTh markers Sox14 and Gbx2 (4).
Here we describe experiments in chicken embryos that were aimed at elucidating the molecular determinants of the prepattern that spatially limits thalamus induction. We initially identified the area of competence for thalamus induction by Shh as the neuroepithelium between the ZLI and the forebrain–midbrain boundary (FMB). We then focused on the neuroepithelium anterior to the ZLI and found that ectopic expression of Irx3 conferred thalamic competence not only to the prethalamus but also to the dorsal telencephalon. Furthermore, we found that Irx3 functions as a transcriptional repressor in mediating thalamic competence and that antagonizing this repressor function impaired thalamic specification.
Subsequently, we focused on the neural tube posterior to the FMB. Activation of the Shh pathway failed to induce thalamic gene expression in the dorsal midbrain (tectum), although Irx3 is expressed there. The paired domain/homeodomain transcription factor Pax6 is expressed throughout the forebrain but not in the midbrain, and one of its early roles is the positioning of the FMB (10–14). Thalamocortical projections are absent and the expression of several regulatory genes is changed in the thalamus of rats and mice lacking Pax6 function, indicating that this gene is essential for thalamogenesis (15, 16). However, Shh from the ZLI down-regulates Pax6 expression in the thalamus at later stages, suggesting that this requirement is transient (4). We hypothesized that Pax6 functions as an additional thalamic competence factor, the absence of which prevents thalamus induction in the midbrain tectum. We found that simultaneous expression of Pax6 and activation of Shh signaling resulted in robust induction of cTh markers in the tectum and, conversely, interfering with endogenous Pax6 function blocked cTh specification. Surprisingly, markers of the rTh were suppressed after overexpression of Pax6 and ectopically up-regulated in cTh territory after inhibition of Pax6, suggesting an additional role for Pax6 in limiting rTh induction.
On the basis of our results, we propose a two-step model for the induction and patterning of thalamic subdivisions: (i) the overlapping expression of Irx3 and Pax6 establishes a prepattern that determines the area of competence for thalamus induction, and (ii) down-regulation of Pax6 by Shh from the ZLI establishes the competence for rTh induction.
Results
Specification of rTh and cTh.
In mouse and zebrafish, characteristic sets of marker genes are differentially expressed in the rTh and cTh (17–19). We performed in situ hybridization (ISH) on chicken embryo brains to establish whether comparable sets of genes are expressed in the rTh and cTh of the avian embryo.
At 5 d of embryonic development (E5), the ZLI is detectable as a peak of Shh expression that protrudes dorsally into the diencephalic alar plate, where it marks the interface between the thalamus posteriorly and the prethalamus anteriorly (Fig. 1A). The homeobox gene Nkx2.2, a bona fide target gene of Shh signaling, is expressed in a narrow band adjacent to the ventral domain of Shh expression throughout the neural tube and on either side of the ZLI (Fig. 1B) (20).
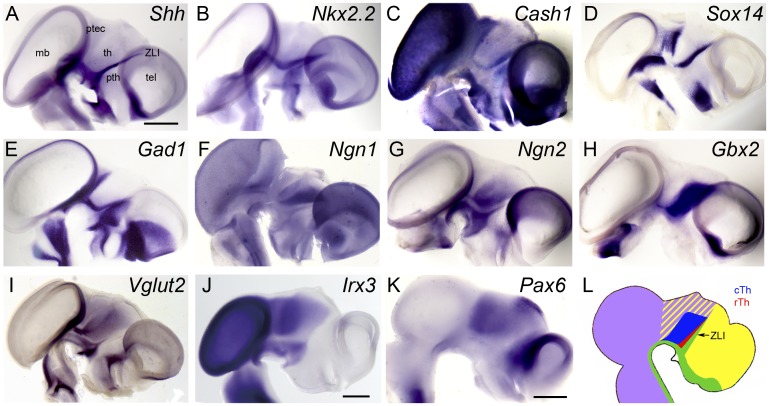
Fig. 1.
Molecular specification of the chick thalamus. (A–K) Lateral views of hemisected E5 (A–I), E4 (J), and E3 (K) chick brains stained by ISH for the expression of (A) Shh, (B) Nkx2.2, (C) Cash1, (D) Sox14, (E) Gad1, (F) Ngn1, (G) Ngn2, (H) Gbx2, (I) Vglut2, (J) Irx3, and (K) Pax6. Anterior points to the right. (Scale bar in A, 1 mm for A–I; scale bars in J and K, 0.5 mm.) (L) Schematic representation of gene expression domains (green, Shh; red, Nkx2.2/Cash1/Sox14/Gad1; blue, Ngn1/Ngn2/Gbx2/Vglut2; purple, Irx3; yellow, Pax6). mb, midbrain; ptec, pretectum; pth, prethalamus; tel, telencephalon; th, thalamus.
In the mouse, rTh progenitors are characterized by the expression of Nkx2.2 and Mash1, encoding a proneural basic helix–loop–helix (bHLH) transcription factor (17, 21). At E5, Cash1, the chick ortholog of Mash1, is expressed in different areas of the developing brain, including the rTh posterior to the ZLI (Fig. 1C). Cash1 expression is transient and has disappeared from the diencephalon at E6 (Fig. S1). From E5 onward, the rTh also expresses Sox14, encoding a high-mobility group transcription factor (Fig. 1D). Finally, rTh neurons express the GABAergic differentiation marker Gad1, encoding glutamate decarboxylase 1, a key enzyme of GABA biosynthesis (Fig. 1E). In summary, chick rTh progenitors express Nkx2.2, Cash1, Sox14, and Gad1 as they differentiate into GABAergic interneurons, comparable to the pattern of gene expression in the mouse rTh (17, 19).
The mouse cTh is characterized by the expression of the proneural bHLH transcription factors Neurogenin1 and Neurogenin2 (Ngn1/2) (17, 21). At E5 both Ngn1 and Ngn2 expression are detectable in the cTh (Fig. 1 F and G). The expression of Ngn1/2 is highly dynamic as it progresses through the cTh primordium in a ventral-to-dorsal wave (Fig. S1), consistent with classic studies describing “neurogenetic gradients” in this brain area (22, 23), but somewhat different from the posterior-to-anterior spread of neurog1 expression observed in the zebrafish thalamus (18).
At early stages of diencephalic development, Gbx2 is weakly expressed in proliferating cTh progenitors (Fig. S1) (24), but it becomes strongly up-regulated in differentiating neurons of this brain region in response to Shh signaling from the ZLI (Fig. 1H) (3–5, 7). Finally, cTh progenitors express the glutamatergic differentiation marker Vglut2 encoding a vesicular glutamate transporter (Fig. 1I). Taken together, the chick cTh gives rise to progenitors that express Ngn1/2, Gbx2, and Vglut2, comparable to what has been described in the mouse (8, 17). Thus, the development of the rTh and cTh is characterized by comparable gene expression signatures in the mouse and chick.
The expression of two prepatterning factors that are known to mediate early neural regionalization overlaps in the area of rTh and cTh induction: Irx3 is expressed caudal to the ZLI in the posterior diencephalon, midbrain, and hindbrain (Fig. 1J and Fig. S1), whereas Pax6 is expressed rostral to the FMB throughout the entire forebrain (Fig. 1K and Fig. S1). In summary, the chick rTh and cTh are induced between the ZLI and the FMB, two boundaries that are characterized by differential expression of Irx3 and Pax6, respectively (Fig. 1L).
Limited Competence for Thalamus Induction.
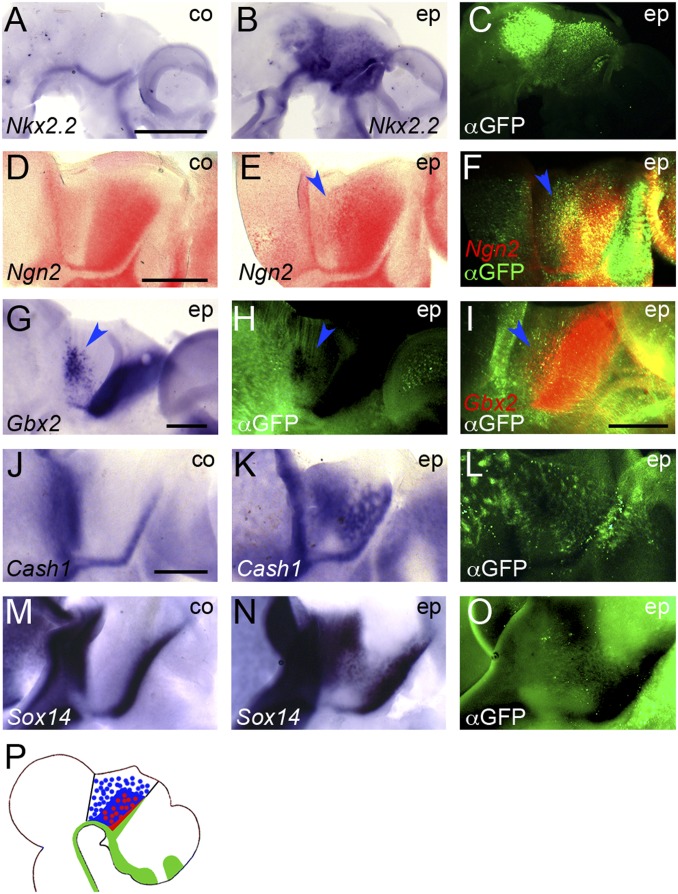
In previous studies, experimental overactivation of Shh signaling led to an expansion and ectopic expression of rTh and cTh markers within the diencephalon posterior to the ZLI (thalamus and pretectum) (3–5, 7, 8). To test the inductive properties of Shh signaling more systematically, we generated an electroporation construct for SmoM2, a constitutively active mutant of the Shh receptor Smoothened (Smo), that is known to strongly activate the Shh signaling pathway in the neural tube of chick and mouse embryos (25, 26). As expected, transfection of this construct by in ovo electroporation into E2.5–3 chick brains resulted in ectopic induction of the bona fide Shh target gene Nkx2.2 at 1 d post electroporation (dpe) in both midbrain and forebrain, on either side of the ZLI (6 of 6; Fig. 2 A–C).
Fig. 2.
Ectopic activation of Shh signaling results in ectopic induction of thalamic markers between the FMB and ZLI. (A–O) Lateral views of hemisected chick brains electroporated (ep) with SmoM2 between E2.5 and E3 and fixed 1 dpe (A–C), 2 dpe (D–F), or 3 dpe (G–O). Anterior points to the right. A, D, J, and M are unelectroporated control halves (co). ISH for Nkx2.2 (A and B), Ngn2 (D–F), Gbx2 (G and I), Cash1 (J and K), and Sox14 (M and N). D–F and I were developed using Fast Red substrate; all others were stained using NBT/BCIP. C, H, L, and O show anti-GFP immunofluorescence (αGFP); F and I show overlay of anti-GFP immunofluorescence and Fast Red fluorescence. [Scale bars in A (for A–C), D (for D–F), I, and J (for J–O), 1 mm; scale bar in G (for G and H) 0.5 mm.] Blue arrowheads point to areas of ectopic induction of Ngn2 (E and F), Gbx2 (G and I), and quenched GFP signal in ectopic Gbx2 area (H). (P) Schematic representation of ectopic induction of rTh (red) and cTh (blue) markers after SmoM2 electroporation.
Markers of the rTh and cTh were also ectopically induced after transfection with SmoM2; however, these inductions were limited to the posterior forebrain between the ZLI and the FMB, despite a wider area being targeted by electroporation (Ngn2: 10 of 12; Gbx2: 10 of 11; Cash1: 5 of 7; Sox14: 6 of 8; Vglut2: 4 of 7; Gad1: 5 of 6; Fig. 2 D–O and Fig. S2 A–G). We showed previously that Pax6 is down-regulated in the thalamic primordium by Shh from the ZLI (4), and transfection with SmoM2 also results in robust down-regulation of Pax6 in the thalamus (5 of 5; Fig. S2 H–J).
The blue precipitate that is generated from nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolylphosphate (NBT/BCIP) in the ISH color reaction quenches the green fluorescent signal marking electroporated cells (e.g., Fig. 2 G and H). To test whether the ectopic induction of thalamic markers occurs in the electroporated cells themselves, we stained some of our ISH specimens using Fast Red, which generates a red fluorescent product (Fig. 2 D–F and I). In areas of ectopic gene expression we found significant overlap between green and red fluorescence, indicating that thalamic induction occurs in cells electroporated with SmoM2 (Fig. 2 F and I). Taken together, these results suggest that Shh signaling is activated throughout the brain by SmoM2; however, the neuroepithelium anterior to the ZLI and that posterior to the FMB lacks competence for thalamus induction by Shh (Fig. 2P).
Repressor Function of Irx3 Mediates Competence for Thalamus Induction.
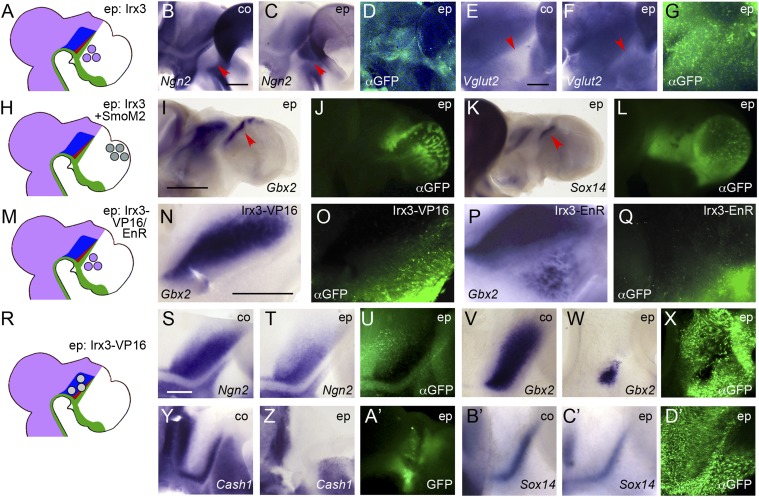
Irx3 is expressed posterior to the ZLI (Fig. 1J) (27) and has previously been demonstrated to endow prethalamic cells with competence to induce Sox14 and Gbx2 in a Shh-dependent manner (4). We found that electroporation of Irx3 into the prethalamus between E2.5 and E3 (Fig. 3A) also results in ectopic induction of Ngn2 (6 of 9; Fig. 3 B–D) and Vglut2 (6 of 8; Fig. 3 E–G) in this area. Cash1 and Gad1 are normally expressed in the prethalamus, a known source of GABAergic neurons (Fig. 1 C and E) (17, 19, 21). Together with our previous findings, these data confirm that Irx3 endows prethalamic cells with competence for the Shh-dependent induction not only of Gbx2 and Sox14 but of a full transcriptional program underlying thalamic differentiation.
Fig. 3.
Irx3 endows cells with thalamic competence in response to Shh signaling. (A, H, M, and R) Schematic representations of electroporation experiments. Lateral views of hemisected (B–G, N–Q, and S–D’) or whole mount (I–L) chick brains electroporated (ep) between E2.5 and E3 with Irx3 into the prethalamus (B–G), with Irx3+SmoM2 into the telencephalon (I–L), with Irx3-VP16 into the prethalamus (N and O), with Irx3-EnR into the prethalamus (P and Q), or with Irx3-VP16 into the thalamus (S–D’) and fixed at 2 dpe (B–D, S–U, and Y–A’) or 3 dpe (E–G, I–L, N–Q, V–X, and B’–D’). Anterior points to the right. B, E, S, V, Y, and B’ are unelectroporated control halves (co). ISH for Ngn2 (B, C, S, and T), Vglut2 (E and F), Gbx2 (I, N, P, V, and W), Sox14 (K, B’, and C’), and Cash1 (Y and Z). D, G, J , L , O , Q , U , X , and D’ show anti-GFP immunofluorescence (αGFP); A’ shows pre-in situ GFP fluorescence. [Scale bars in B (for B–D), E (for E–G), and S (for S–D’), 0.25 mm; scale bar in I (for I–L), 1 mm; scale bar in N (for N–Q), 0.5 mm. Red arrowheads point at prethalamus in B, C, E, and F and at domains of ectopic gene expression in I and K.
If Irx3 mediates thalamic competence, one would also expect ectopic induction of thalamus markers after ectopic expression of Irx3 and simultaneous activation of the Shh pathway in the otherwise Irx3-negative telencephalon. Because Cash1, Gad1, Ngn1/2, and Vglut2 are normally expressed in parts of the telencephalon (Fig. 1 C and E–G and Fig. S1), we focused on analyzing whether the cTh and rTh markers Gbx2 and Sox14 are induced there by Irx3. Because the pallium develops at some distance from Shh-expressing signaling centers, we cotransfected Irx3 with SmoM2 to mimic the inductive activity of the ZLI (Fig. 3H). Electroporation of neither Irx3 nor SmoM2 individually led to induction of Gbx2 (0 of 15) or Sox14 (0 of 15); however, combinatorial expression of both frequently resulted in ectopic Gbx2 and Sox14-positive cells in the telencephalon (14 of 17 and 9 of 15; Fig. 3 I–L). Interestingly, such cells were predominantly found in the dorsalmost pallium, suggesting that thalamus induction is blocked in the ventral telencephalon—either owing to the lack of another competence factor or to the presence of an inhibitory factor.
If Irx3 is required to mediate thalamic competence, inhibiting its function should result in defects of rTh and cTh specification. We tried electroporating shRNA-producing plasmids targeting Irx3 mRNA; however, these attempts failed to produce any significant changes in thalamic gene expression. Because we found that at least four of the six Irx genes are expressed in the presumptive thalamus (Fig. S1), we speculate that the failure to obtain an effect with Irx3 shRNAs could be due to compensatory effects of other members of the Irx family.
We reasoned that a dominant-negative approach could achieve a broader inhibitory effect on Irx function because it is likely to outcompete not only Irx3 but also other Irx factors binding to the same recognition sites. Thus, we generated fusion constructs of Irx3’s DNA-binding homeodomain with either the VP16 transactivation domain or the Engrailed repressor (EnR) domain and tested the ability of these chimeric proteins to confer thalamic competence to the prethalamus (Fig. 3M). Electroporation of Irx3-EnR, but not of Irx3-VP16, resulted in ectopic induction of Gbx2 in the prethalamus (5 of 6 versus 0 of 8; Fig. 3 N–Q), demonstrating that Irx3 functions as a transcriptional repressor in mediating thalamic competence.
Because Irx3 acts as a repressor, we expected Irx3-VP16 to antagonize endogenous Irx3 function in a dominant-negative fashion (Fig. 3R). Indeed, electroporation of Irx3-VP16 into the thalamic primordium disrupted regional gene expression in the cTh and rTh (Ngn2: 5 of 10; Gbx2: 6 of 14; Vglut2: 3 of 3; Cash1: 9 of 12; Sox14: 3 of 5; Gad1: 3 of 3; Fig. 3 S–D’ and Fig. S2 K–P). In more saturating Irx3-VP16 electroporations, the thalamic primordium was severely reduced, and markers of the rTh were often completely absent (e.g., Fig. 3 Y–A’). Interestingly, full-length Irx3 fused to VP16 failed to elicit comparable effects. These results indicate that Irx3 is not only sufficient to endow cells anterior to the ZLI with thalamic competence but also required for the specification of rTh and cTh.
Pax6 Mediates Competence for cTh Induction by Shh.
The midbrain widely expresses Irx3, but no induction of thalamic gene expression is observed in this area after activation of Shh signaling (Fig. 2 D–P). That the competence for thalamus induction is limited to the posterior forebrain is particularly evident in Fig. 2G, where—despite widespread electroporation of SmoM2 (Fig. 2H)—ectopic Gbx2 expression is only found in the pretectum with its posterior limit precisely outlining the FMB. Pax6 is broadly expressed in the forebrain from early developmental stages onward (Fig. 1K and Fig. S1), and its posterior limit marks the FMB. Thus, we speculated that Pax6 could function as an additional competence factor, the absence of which results in lack of thalamus induction in the tectum.
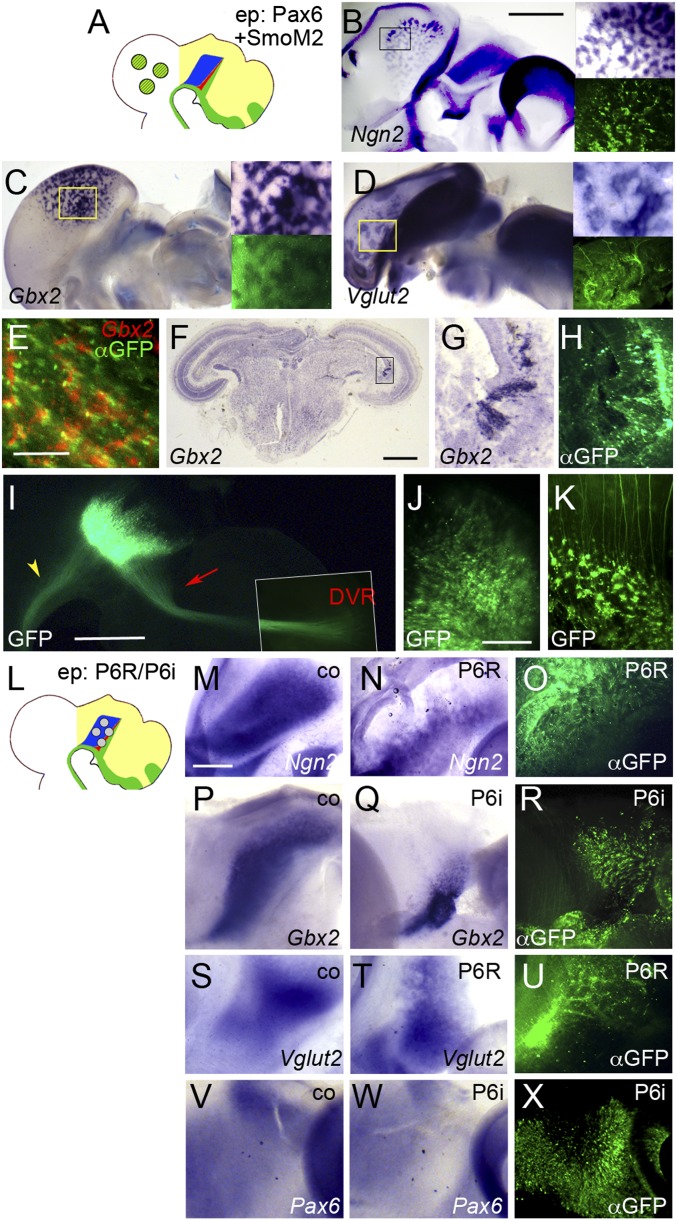
To test this hypothesis, we coelectroporated Pax6 and SmoM2 into the chick tectum between E2.5 and E3 (Fig. 4A) and analyzed the expression of thalamic marker genes between 1 and 3 dpe. Transfection of tectal cells with Pax6 did not result in induction of Ngn2 (0 of 12; Fig. S3A), whereas weak induction was detected after electroporation of SmoM2 (7 of 10; Fig. S3B). In contrast, robust Ngn2 induction was observed after cotransfection of Pax6 and SmoM2 (12 of 14; Fig. 4B and Fig. S3C). Electroporation of either SmoM2 or Pax6 individually did not lead to induction of Gbx2 (0 of 18 and 0 of 19) or Vglut2 (0 of 4 and 0 of 7), but combinatorial expression of both Pax6 and SmoM2 resulted in abundant induction of Gbx2 and Vglut2-positive cells (46 of 56 and 8 of 9; Fig. 4 C and D). The induction of these markers followed the temporal progression of normal cTh differentiation, with Ngn2-positive cells becoming detectable at 1 dpe and Gbx2 and Vglut2-positve cells accumulating at 3 dpe. To test whether cTh progenitors are induced in the cells transfected with Pax6 and SmoM2, or non-cell autonomously via an indirect mechanism, we performed ISH for Gbx2 using Fast Red. We found significant overlap between green and red fluorescence in such embryos, suggesting that ectopic induction of Gbx2 in the tectum occurs in clusters of electroporated cells (Fig. 4E; note that the red fluorescent areas appear to be wider than the green signals, possibly because the Fast Red precipitate is somewhat diffusible).
Fig. 4.
Pax6 mediates competence for cTh induction by Shh. (A and L) Schematic representations of electroporation experiments. (B–K and M–X) Lateral views of hemisected chick brains (B–E, I–K, M–X) and transverse section through chick brain (F–H) electroporated (ep) into the midbrain (B–H, J, and K) or thalamus (I and M–X) between E2.5 and E3 with Pax6+SmoM2 (B–H and K), GFP alone (I and J), Pax6-EnR (P6R; M–O and S–U) or Pax6-RNAi (P6i; P–R and V–X) and fixed at 1 dpe (V–X), 2 dpe (M–O), 3 dpe (B–E, I–K, and P–U), or 6 dpe (F–H). Anterior points to the right. (M, P, S, and V) Unelectroporated control halves (co). ISH for Ngn2 (B, M, and N), Gbx2 (C, E–G, P, and Q), Vglut2 (D, S, and T) and Pax6 (V and W). (Insets in B–D) Magnification of boxed area in main panel (Upper Right) and corresponding anti-GFP immunofluorescence (Lower Right). (E) Overlay of anti-GFP immunofluorescence (αGFP) and Fast Red fluorescence (Gbx2). (G and H) Magnification of boxed area in F. (H, O, R, and U) Anti-GFP immunofluorescence; (I–K) GFP fluorescence. Note that I is a composite picture; the Inset shows the termination zone at higher contrast. [Scale bars in B (for B–D), F, and I, 1 mm; scale bar in E, 0.1 mm; scale bar in J (for J and K), 0.2 mm; scale bar in M (for M–X), 0.5 mm.] Red arrow and yellow arrowhead in I point at projection to the DVR and mlf, respectively.
Pax6, Ngn2, Gbx2, and Vglut2 are also expressed in a subset of hindbrain neurons. To rule out that the inductions observed in our cotransfections represent an induction of hindbrain rather than thalamus identity, we analyzed the expression of the forebrain/midbrain marker Otx2. Simultaneous expression of Pax6 and activation of Shh signaling resulted in mild up-regulation of Otx2 expression at 2 dpe (6 of 6; Fig. S3D). At 3 dpe, Otx2 becomes down-regulated in electroporated cells; however, it is also down-regulated in the thalamus at this stage (3 of 3; Fig. S3E). This expression dynamic supports the view that the induced Ngn2/Gbx2/Vglut2-positive cells are of forebrain identity. Our results indicate that Pax6 endows tectal cells with competence to form cTh neurons in response to Shh.
Notably, the ectopically induced cTh neurons tend to segregate into clusters, presumably because the structural organization of the thalamus differs from the layered architecture of the tectum (Fig. 4 B–D) (5). To assess the topological effects of the midbrain–cTh transformation, we electroporated Pax6 and SmoM2 into the midbrain between E2.5 and E3 and fixed the embryos at 6 dpe when tectal layers had become distinguishable. Gbx2-positive clusters are still clearly detectable in sections through such midbrains and are found close to the ventricular surface, possibly between the subventricular zone and the stratum griseum centrale (Fig. 4 F–H).
In the mouse, projections from the thalamus innervate layer 4 of the neocortex (28). After electroporation of GFP into the chick thalamus at E3 we observed a distinctive axon bundle leading from the thalamus to the dorsoventricular ridge (DVR) of the pallium at 3 dpe—the likely counterpart of the thalamocortical projection in the mouse (29, 30). Furthermore, we detected some posterior projections to the hindbrain and spinal cord that are likely to contribute to the medial longitudinal fascicle (mlf; Fig. 4I) (31). Previously Pax6 has been implicated in axonogenesis in the forebrain (11, 14, 28, 32, 33), prompting us to investigate whether the ectopically induced cTh neurons in the midbrain form thalamus-like projections. GFP electroporations into the tectum led to only a few posterior projections joining the lateral longitudinal fascicle, whereas ectopic cTh neurons induced by coexpression of Pax6 and SmoM2 in the midbrain formed abnormal projections growing in different directions depending on the specific location of their cell bodies in the tectum (Fig. 4 J and K). We speculate that these cells may have acquired an enhanced ability to form long-range projections similar to neurons of the glutamatergic thalamus, but that these axons fail to project to the pallium because they are exposed to guidance cues different from those in the thalamus.
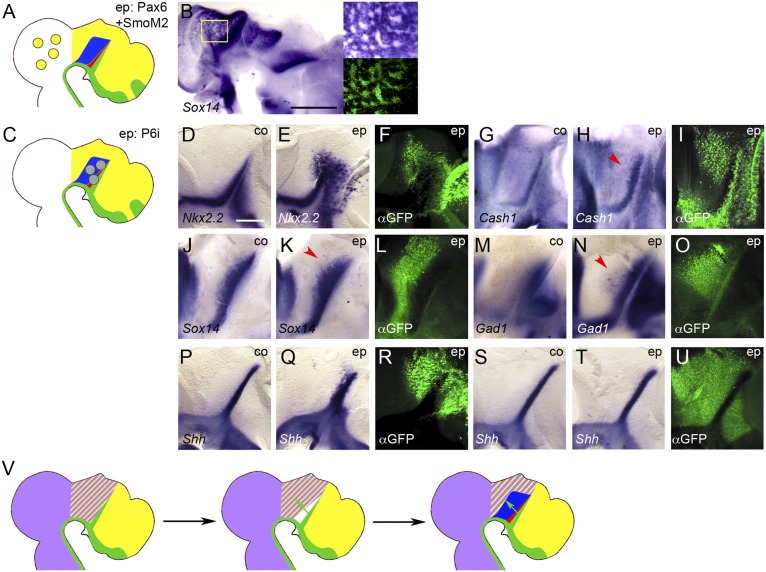
If Pax6 mediates cTh competence, interfering with its endogenous function should impair thalamogenesis (Fig. 4L). Indeed, electroporation of a Pax6-Engrailed repressor fusion construct (Pax6-EnR) that is known to act as a dominant-negative form of Pax6 (13) resulted in down-regulation of Ngn2, Gbx2, and Vglut2 in the cTh (16 of 16, 24 of 25, and 4 of 4; Fig. 4 M–O and S–U). We confirmed this result by electroporating an RNAi plasmid directed against Pax6 (34), which also resulted in down-regulation of Gbx2 in the cTh (11 of 17; Fig. 4 P–R). As previously demonstrated in the spinal cord (34), electroporation of the Pax6-RNAi plasmid also leads to down-regulation of Pax6 mRNA in the presumptive thalamus, confirming the efficiency of this knockdown approach (5 of 7; Fig. 4 V–X). Taken together, these results indicate that Pax6 functions as a mediator of cTh competence.
Pax6 Antagonizes rTh Induction.
Markers of the rTh—Cash1, Sox14, and Gad1—are also expressed in the midbrain (Fig. 1C and Fig. S1). We speculated that if Pax6 functions as a general thalamic competence factor, simultaneous expression of Pax6 and SmoM2 might boost the expression of these markers in the tectum (Fig. 5A). Surprisingly, we found that their expression was down-regulated after such electroporations (Cash1: 9 of 10; Sox14: 12 of 13; Gad1: 10 of 10; Fig. 5B). Together with the Shh-dependent down-regulation of Pax6 adjacent to the ZLI (Fig. S2 H–J) (4), these results suggested that Pax6 might antagonize rTh induction. Indeed, forcing Pax6 expression throughout the diencephalon by in ovo electroporation frequently resulted in down-regulation of rTh markers (Cash1: 9 of 10; Sox14: 12 of 13; Gad1: 10 of 10; Fig. S3 F–N), suggesting that the down-regulation of Pax6 posterior to the ZLI is required for rTh induction.
Fig. 5.
Pax6 antagonizes rTh formation. (A and C) Schematic representations of electroporation experiments. (B and D–U) Lateral views of hemisected chick brains electroporated (ep) into the midbrain with Pax6+SmoM2 (B) or thalamus with Pax6-RNAi (P6i; D–U) at E3 (B, D–O, and S–U) or E1.5 (P–R) and fixed at 3 dpe (B and M–O), 1 dpe (D–F and S-U) or 2 dpe (G–L and P–R). Anterior points to the right. (D, G, J, M, P, and S) Unelectroporated control halves (co). ISH for Sox14 (B, J, and K), Nkx2.2 (D and E), Cash1 (G and H), Gad1 (M and N), and Shh (P, Q, S, and T). (Insets in A) Magnification of boxed area in main panel (Upper Right) and corresponding anti-GFP immunofluorescence (Lower Right). (F, I, L, O, R, and U) Anti-GFP immunofluorescence (αGFP). [Scale bar in B, 1 mm; scale bar in D (for D–U), 0.25 mm. Red arrowheads point to up-regulation and expansion of rTh markers in H, K, and N. (V) Model for the induction and regionalisation of the thalamus: Irx3 (purple) is expressed posterior to the ZLI, Pax6 (yellow) anterior to the FMB; the overlap of these expression domains defines the area of thalamic competence; Shh signaling from the ZLI (green arrows) down-regulates Pax6 in the vicinity of the ZLI, thereby allowing for the induction of the rTh (red) at higher doses and of the cTh (blue) at lower doses of Shh.
If Pax6 needs to be suppressed for the establishment of rTh identity, is Pax6 required to prevent ectopic rTh induction in the cTh domain? In the spinal cord, Pax6 represses the expression of Nkx2.2, which mediates ventral patterning by Shh (35, 36). The rTh is derived from an Nkx2.2-positive progenitor region posterior to the ZLI, and Nkx2.2 expression is expanded in the thalamus of Pax6−/− mouse embryos (16). We found that blocking endogenous Pax6 function at E3 using Pax6-RNAi (Fig. 5C) resulted in ectopic expression of Nkx2.2 throughout the brain, consistent with the spinal cord and mouse forebrain data (13 of 19; Fig. 5 D–F). Markers of rTh identity are also expanded into the cTh area under such conditions, although the effects are more subtle than for Nkx2.2 (Cash1: 2 of 4; Sox14: 3 of 7; Gad1: 4 of 7; Fig. 5 G–O). We found similar effects on rTh gene expression after electroporation of the Pax6-EnR construct, albeit at lower frequency.
Previous studies indicated the rTh is induced by high levels, whereas the cTh requires lower levels of Shh (3). Thus, it is possible that the effect of Pax6 on the formation of these two domains is mediated via a direct effect on the Shh-producing cells in the ZLI. Indeed, we found that electroporation of Pax6 efficiently repressed Shh in the ZLI and in other areas of the forebrain (11 of 11; Fig. S3 O–Q). Electroporating Pax6-RNAi at E2 resulted in a noticeable shortening and broadening of the Shh expression domain in the ZLI, consistent with the phenotype of the Pax6 mutant mouse (6 of 10; Fig. 5 P–R) (16). However, transfection of the diencephalon with Pax6-RNAi at E3 never led to significant changes in Shh expression or morphological alterations of the ZLI that could account for the widening of the rTh in such embryos (0 of 9; Fig. 5 S–U). Thus, Pax6 is likely to promote cTh and counteract rTh formation at the level of the signal-receiving cells.
If Pax6 antagonizes rTh identity, why can we induce ectopic expression of Sox14 by electroporating Irx3 and SmoM2 into the Pax6-positive telencephalon? We found that Pax6 expression becomes suppressed in telencephalic areas transfected with Irx3 and SmoM2 (5 of 5; Fig. S3 R and S), comparable to the down-regulation of Pax6 by ZLI-derived Shh in the Irx3-positive thalamus. We tested our model further by analyzing Gbx2 and Sox14 expression in telencephalons simultaneously transfected with Irx3, SmoM2, and Pax6. Whereas no change in Gbx2 induction was found, Sox14 induction was noticeably decreased under these conditions (5 of 5 vs. 2 of 6 and far less Sox14+ cells in such embryos; Fig. S3 T–W).
We conclude that Pax6 suppresses genes characteristic of rTh specification not only in the thalamus but also in the midbrain and telencephalon.
Discussion
The molecular specification of the rTh and cTh has been described in some detail in the mouse embryo (17, 19), and we have confirmed here that orthologous sets of marker genes are expressed in the rTh and cTh of the chick, indicating molecular conservation of thalamic patterning in amniotes. We then defined the area of competence for the induction of these markers by Shh as the neuroepithelium between the ZLI and the FMB, suggesting the existence of a prepattern that regulates how cells respond to Shh.
Irx3 has previously been suggested to mediate differential competence around the ZLI. Here, we found that misexpression of Irx3 in the prethalamus results in ectopic induction not only of Gbx2 and Sox14 but of a range of markers characteristic of thalamus differentiation. We were also able to induce rTh and cTh markers in the dorsal telencephalon by simultaneous expression of Irx3 and activation of Shh signaling. No such inductions were observed in the ventral telencephalon, suggesting either that another, as yet unidentified, competence factor is lacking or that an inhibitor of thalamus induction is active there. One possible candidate is the homeodomain transcription factor Six3 that is known to antagonize Irx3 and has been suggested to establish the identity of the neuroepithelium rostral to the ZLI (27). Six3 is initially expressed widely throughout the forebrain and then becomes progressively restricted to the ventral telencephalon, so it is conceivable that only the dorsal-most cells of the telencephalon—where Six3 is first down-regulated—have sufficiently low levels to allow for thalamus induction.
At least four members of the Irx family are expressed in the developing thalamus. This points toward a high level of redundancy and may explain why our attempts to attenuate Irx3 function using shRNAs failed. In the mouse and zebrafish, Irx1 and irx1b, respectively, have been suggested to play a role similar to that of chick Irx3 (37, 38). In the chick, Irx1 is expressed less widely than Irx3; however, it is found in the thalamus between E3 and E5 and may therefore partially compensate for a loss of Irx3 (Fig. S1). Furthermore, both Irx2 and Irx5 are also found in this area. Our dominant-negative approach is likely to have circumvented compensatory effects by other members of the Irx family.
Vertebrate Irx genes are organized in two clusters: the IrxA cluster containing Irx1, Irx2, and Irx4 and the IrxB cluster containing Irx3, Irx5, and Irx6 (39). Genes in equivalent positions within these clusters (Irx1/Irx3, Irx2/Irx5, Irx4/Irx6) are paralogues and therefore most similar; however, partial functional compensation has been described even for nonparalogous Irx genes, for example in heart formation (40). Defects in brain patterning have been observed in the Fused toes mouse mutant that carries a chromosomal deletion eliminating the IrxB cluster (41); however, thalamic specification seems relatively normal in these mice, consistent with Irx1 being the major mediator of thalamic competence in rodents.
Irx proteins contain an acidic activation domain, suggesting that they may function as transcriptional activators (40, 42). However, Xenopus irx1 was shown to operate as a transcriptional repressor in neural plate specification in frogs (39). We found that chick Irx3 exerts repressor function in mediating thalamic competence and that thalamic specification is impaired after electroporation of a VP16-Irx3 fusion protein. In these experiments, only a construct that contained the DNA-binding homeodomain of Irx3 but lacked the IRO motif and most of the linker region led to a dominant-negative effect, whereas no consistent results were obtained with a full-length Irx3-VP16 fusion construct. We speculate that the repressor function of Irx3 resides in its C terminus, the deletion of which is required to achieve a dominant-negative effect.
Irx3 is expressed throughout the midbrain, but activation of Shh signaling does not result in thalamus induction there, implying an additional mechanism of competence restriction around the FMB. Pax6 is expressed throughout the forebrain from early developmental stages onward and has been implicated in multiple processes, including forebrain patterning, FMB positioning, boundary formation, and axonogenesis (14). At the time of thalamus induction, the posterior border of Pax6 expression precisely marks the FMB. Using an experimental approach comparable to the one we used for Irx3, we demonstrated that Pax6 also functions as a thalamic competence factor that can mediate cTh induction in response to Shh in the tectum and that it is required for cTh formation in the diencephalon. Ectopically induced thalamic neurons in the tectum form clusters that fail to integrate into tectal layers and unusual long-range projections, but these axons fail to form a bundle that projects to the DVR like neurons of the thalamus proper, presumably because they are in the wrong microenvironment.
Notably, rTh markers were down-regulated after overexpression of Pax6 and were expanded as a result of attenuating its function, indicating that Pax6 promotes cTh at the expense of rTh identity, consistent with the down-regulation of Pax6 posterior to the ZLI. Both Shh and Nkx2.2 expression are expanded in the thalamus of Pax6−/− mutant mice (16), suggesting that the regulatory effect of Pax6 on the formation of the rTh and cTh could be mediated via differences in Shh signaling from the ZLI. Indeed, we found that forcing the expression of Pax6 throughout the diencephalon efficiently represses Shh (Fig. S3 O–Q), that Nkx2.2 is ectopically expressed after Pax6 inhibition (Fig. 5 D–F), and that the ZLI is shorter and broader in embryos electroporated with Pax6-RNAi at E2 (Fig. 5 P–R). However, no significant changes in Shh expression and ZLI morphology were observed after attenuation of Pax6 function at E3—an experimental condition that frequently results in a widening of the rTh domain (Fig. 5 S–U). Thus, it seems that the early function of Pax6 in limiting the ZLI territory has become redundant at thalamic patterning stages.
Our results indicate that Pax6 promotes cTh at the cost of rTh identity, but they raise the question of whether the induction of the rTh is mediated exclusively via down-regulation of Pax6 (disinhibition only) or whether there is a Pax6-independent function of Shh in establishing rTh identity (disinhibition plus induction). Even in electroporation experiments in which Pax6-RNAi was widely transfected throughout the thalamus, markers of the rTh were expanded but remained confined to the anterior portion of the thalamus close to the ZLI (Fig. 5 G–O). This suggests that loss of Pax6 function alone is not sufficient and that Pax6-negative cells still require Shh pathway activation for the induction of the rTh.
On the basis of our results, we propose a two-step model for thalamus induction by Shh (Fig. 5V): (i) the overlapping expression of Irx3 and Pax6 determines the area of competence for the induction of the cTh, and (ii) the down-regulation of Pax6 by Shh from the ZLI regulates the balance between cTh and rTh formation. It is possible that these two steps occur sequentially, such that general competence for thalamus induction is initially established by Irx3 and Pax6, and the rTh domain is subsequently specified. Alternatively, both steps may occur simultaneously. In all likelihood, many more factors contribute to setting up competence areas in the developing brain—as indicated by our observation that the ventral telencephalon seems to be comparably refractory for thalamic induction.
This study adds Pax6 to a growing list of factors that regulate the balance between glutamatergic and GABAergic neurogenesis in the thalamus (21, 43–45), and more generally, it highlights that the prepattern that determines the response to an organizer signal is not static but may itself be regulated by this signal. Thus, extrinsic and intrinsic factors continuously interact during central nervous system development, resulting in the progressive restriction of cell fate that underlies the generation of the multitude of cell types characteristic of the vertebrate brain.
Materials and Methods
In Ovo Electroporation, ISH, Immunofluorescence.
Fertilized hen’s eggs were obtained from Henry Stewart & Co. and were incubated up to desired stages at 38 °C in a humidified chamber. In ovo electroporation, ISH, and anti-GFP immunofluorescence were performed as described before (4). Plasmids were electroporated at either 1 mg/mL or 2 mg/mL (VP16-Irx3), and a GFP expressing plasmid was always coelectroporated to allow for localization of the electroporated area. E10 embryos were embedded in optimal cutting temperature (OCT) medium, 20-μm sections were prepared on a Zeiss cryostat, and ISH was performed using standard techniques (19).
Electroporation Constructs.
Human Smoothened M2 was subcloned into pCAβ-IRES-eGFPm5 (J. Gilthorpe, Umea University, Umea, Sweden). For VP16-Irx3 and EnR-Irx3, the chick Irx3 homeobox and its conserved flanking region (approximately 240 bp) were subcloned into shuttle vectors containing the VP16 transactivation and Engrailed repressor domain, respectively. The fused cassettes were then subcloned into pCIG (a derivative of pCAGGS containing IRES-GFP; A. McMahon, Harvard University, Cambridge, MA). The full-length chick Irx3-VP16 construct was generated by inserting the VP16 cassette into pCAGGS-cIrx3 (T. Ogura, Tohoku University, Sendai, Japan) (4). The Pax6-RNAi electroporation construct (ARK Genomics) expresses a short hairpin RNA directed against the sequence GGCACCACTTCCACAGGTCTCA from a microRNA operon expression cassette using the chick U6 promoter; this construct was previously shown to efficiently knockdown Pax6 mRNA after electroporation into the chick spinal cord (34).
Supplementary Material
Acknowledgments
We thank Genentech for SmoM2, ARK Genomics for the Pax6-RNAi plasmid, T. Ogura, N. Osumi, and D. Sela-Donenfeld for sharing reagents, and A. Graham and two anonymous reviewers for helpful comments on the manuscript. This work was supported by Medical Research Council Grants G9027130 and G0601064. E.R. was supported by a Biotechnology and Biological Sciences Research Council studentship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304311110/-/DCSupplemental.
References
- 1.Spemann H. Über Correlationen in der Entwicklung des Auges. Verh Anat Ges. 1901;15:61–79. [Google Scholar]
- 2.Kiecker C, Lumsden A. The role of organizers in patterning the nervous system. Annu Rev Neurosci. 2012;35:347–367. doi: 10.1146/annurev-neuro-062111-150543. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto-Torii K, et al. Differential activities of Sonic hedgehog mediated by Gli transcription factors define distinct neuronal subtypes in the dorsal thalamus. Mech Dev. 2003;120(10):1097–1111. doi: 10.1016/j.mod.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7(11):1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- 5.Vieira C, Garda AL, Shimamura K, Martinez S. Thalamic development induced by Shh in the chick embryo. Dev Biol. 2005;284(2):351–363. doi: 10.1016/j.ydbio.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: The multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(10):772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 7.Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development. 2006;133(5):855–864. doi: 10.1242/dev.02248. [DOI] [PubMed] [Google Scholar]
- 8.Vue TY, et al. Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J Neurosci. 2009;29(14):4484–4497. doi: 10.1523/JNEUROSCI.0656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong Y, et al. Spatial and temporal requirements for sonic hedgehog in the regulation of thalamic interneuron identity. Development. 2011;138(3):531–541. doi: 10.1242/dev.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996;122(11):3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- 11.Mastick GS, Davis NM, Andrew GL, Easter SSJ., Jr Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997;124(10):1985–1997. doi: 10.1242/dev.124.10.1985. [DOI] [PubMed] [Google Scholar]
- 12.Stoykova A, Götz M, Gruss P, Price J. Pax6-dependent regulation of adhesive patterning, R-cadherin expression and boundary formation in developing forebrain. Development. 1997;124(19):3765–3777. doi: 10.1242/dev.124.19.3765. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga E, Araki I, Nakamura H. Pax6 defines the di-mesencephalic boundary by repressing En1 and Pax2. Development. 2000;127(11):2357–2365. doi: 10.1242/dev.127.11.2357. [DOI] [PubMed] [Google Scholar]
- 14.Georgala PA, Carr CB, Price DJ. The role of Pax6 in forebrain development. Dev Neurobiol. 2011;71(8):690–709. doi: 10.1002/dneu.20895. [DOI] [PubMed] [Google Scholar]
- 15.Kawano H, et al. Pax-6 is required for thalamocortical pathway formation in fetal rats. J Comp Neurol. 1999;408(2):147–160. doi: 10.1002/(sici)1096-9861(19990531)408:2<147::aid-cne1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Pratt T, et al. A role for Pax6 in the normal development of dorsal thalamus and its cortical connections. Development. 2000;127(23):5167–5178. doi: 10.1242/dev.127.23.5167. [DOI] [PubMed] [Google Scholar]
- 17.Vue TY, et al. Characterization of progenitor domains in the developing mouse thalamus. J Comp Neurol. 2007;505(1):73–91. doi: 10.1002/cne.21467. [DOI] [PubMed] [Google Scholar]
- 18.Scholpp S, et al. Her6 regulates the neurogenetic gradient and neuronal identity in the thalamus. Proc Natl Acad Sci USA. 2009;106(47):19895–19900. doi: 10.1073/pnas.0910894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delogu A, et al. Subcortical visual shell nuclei targeted by ipRGCs develop from a Sox14+-GABAergic progenitor and require Sox14 to regulate daily activity rhythms. Neuron. 2012;75(4):648–662. doi: 10.1016/j.neuron.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura K, Miura H, Yanazawa M, Miyashita T, Kato K. Expression patterns of Brx1 (Rieg gene), Sonic hedgehog, Nkx2.2, Dlx1 and Arx during zona limitans intrathalamica and embryonic ventral lateral geniculate nuclear formation. Mech Dev. 1997;67(1):83–96. doi: 10.1016/s0925-4773(97)00110-x. [DOI] [PubMed] [Google Scholar]
- 21.Puelles E, et al. Otx2 controls identity and fate of glutamatergic progenitors of the thalamus by repressing GABAergic differentiation. J Neurosci. 2006;26(22):5955–5964. doi: 10.1523/JNEUROSCI.1097-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angevine JBJ., Jr Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol. 1970;139(2):129–187. doi: 10.1002/cne.901390202. [DOI] [PubMed] [Google Scholar]
- 23.McAllister JP II, Das GD. Neurogenesis in the epithalamus, dorsal thalamus and ventral thalamus of the rat: An autoradiographic and cytological study. J Comp Neurol. 1977;172(4):647–686. doi: 10.1002/cne.901720407. [DOI] [PubMed] [Google Scholar]
- 24.Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130(23):5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- 25.Hynes M, et al. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat Neurosci. 2000;3(1):41–46. doi: 10.1038/71114. [DOI] [PubMed] [Google Scholar]
- 26.Ribes V, et al. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010;24(11):1186–1200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi D, et al. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129(1):83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- 28.Molnár Z, Garel S, López-Bendito G, Maness P, Price DJ. Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. Eur J Neurosci. 2012;35(10):1573–1585. doi: 10.1111/j.1460-9568.2012.08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiner A, et al. Avian Brain Nomenclature Forum Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473(3):377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci USA. 2012;109(42):16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware M, Schubert FR. Development of the early axon scaffold in the rostral brain of the chick embryo. J Anat. 2011;219(2):203–216. doi: 10.1111/j.1469-7580.2011.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews GL, Mastick GS. R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci. 2003;23(30):9873–9880. doi: 10.1523/JNEUROSCI.23-30-09873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nural HF, Mastick GS. Pax6 guides a relay of pioneer longitudinal axons in the embryonic mouse forebrain. J Comp Neurol. 2004;479(4):399–409. doi: 10.1002/cne.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das RM, et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev Biol. 2006;294(2):554–563. doi: 10.1016/j.ydbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Ericson J, et al. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90(1):169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 36.Briscoe J, et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398(6728):622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 37.Hirata T, et al. Zinc-finger genes Fez and Fez-like function in the establishment of diencephalon subdivisions. Development. 2006;133(20):3993–4004. doi: 10.1242/dev.02585. [DOI] [PubMed] [Google Scholar]
- 38.Scholpp S, et al. Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development. 2007;134(17):3167–3176. doi: 10.1242/dev.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Skarmeta JL, Modolell J. Iroquois genes: Genomic organization and function in vertebrate neural development. Curr Opin Genet Dev. 2002;12(4):403–408. doi: 10.1016/s0959-437x(02)00317-9. [DOI] [PubMed] [Google Scholar]
- 40.Gaborit N, et al. Cooperative and antagonistic roles for Irx3 and Irx5 in cardiac morphogenesis and postnatal physiology. Development. 2012;139(21):4007–4019. doi: 10.1242/dev.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anselme I, Laclef C, Lanaud M, Rüther U, Schneider-Maunoury S. Defects in brain patterning and head morphogenesis in the mouse mutant Fused toes. Dev Biol. 2007;304(1):208–220. doi: 10.1016/j.ydbio.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Bürglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25(21):4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kataoka A, Shimogori T. Fgf8 controls regional identity in the developing thalamus. Development. 2008;135(17):2873–2881. doi: 10.1242/dev.021618. [DOI] [PubMed] [Google Scholar]
- 44.Bluske KK, et al. β-Catenin signaling specifies progenitor cell identity in parallel with Shh signaling in the developing mammalian thalamus. Development. 2012;139(15):2692–2702. doi: 10.1242/dev.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virolainen SM, Achim K, Peltopuro P, Salminen M, Partanen J. Transcriptional regulatory mechanisms underlying the GABAergic neuron fate in different diencephalic prosomeres. Development. 2012;139(20):3795–3805. doi: 10.1242/dev.075192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.