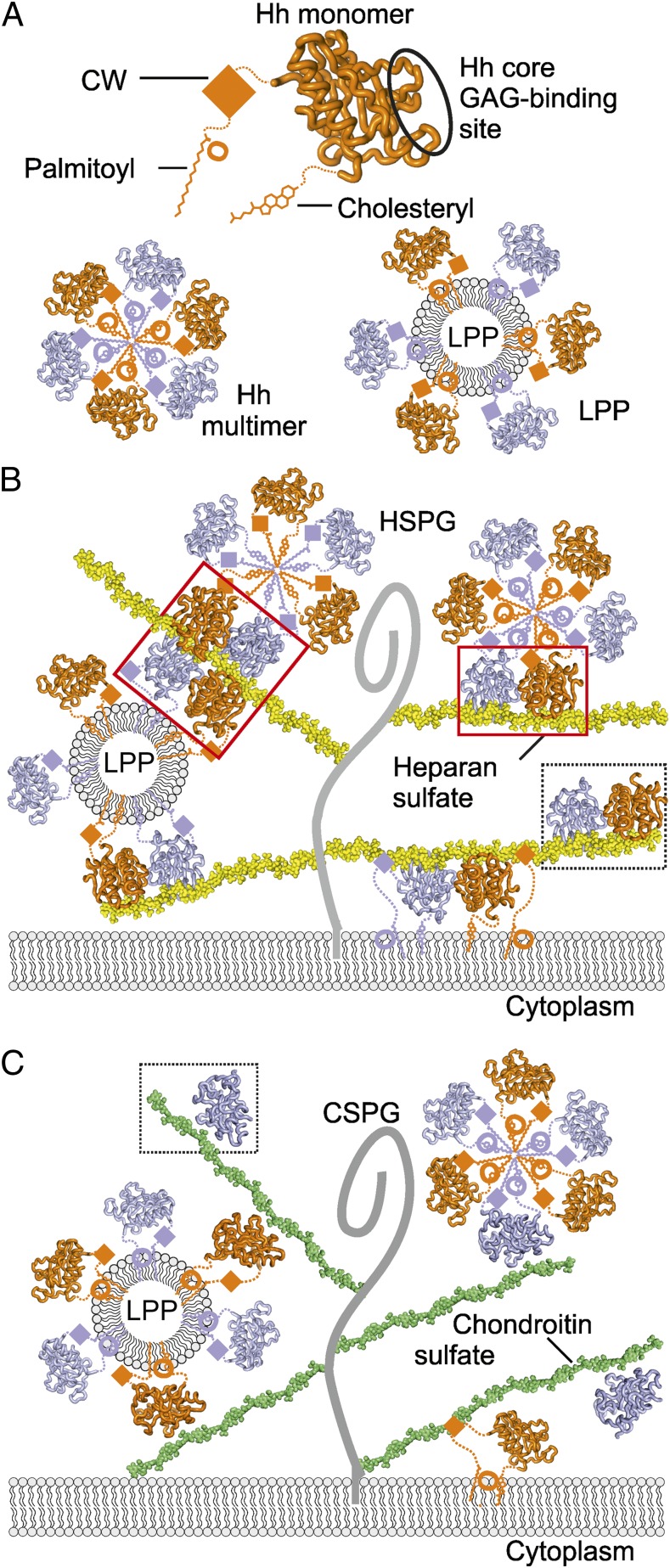
Fig. 5.
Model of Hh–GAG interactions at the cell surface. (A) The Hh monomer (orange) is covalently linked to palmitoyl and cholesteryl moieties at the N- and C-termini, respectively. CW is located between the palmitoyl and Hh core domain. Our identified Hh core GAG-binding site is circled. Hh monomers assemble into homomultimers by burying lipid moieties within the hydrophobic core (Lower Left) or as part of lipoprotein particles (LPP) (Lower Right). (B) Different monomeric and oligomeric forms of Hh bind to HS chains (yellow) linked to the stalk regions of HSPGs (gray curve). Both CW and the Hh core GAG-binding site mediate Hh binding to HS. High degree of HS sulfation mediates HS-dependent Hh oligomerization. HS-induced tetramer and dimer are shown in red boxes, the Shh–heparin complex observed in the crystallographic asymmetric unit, in a dashed box. (C) Similarly to HS, CS chains (green) bind to multiple forms of Hh (monomers or lipid-induced oligomers). However, in contrast to HS, the less sulfated CS provides a platform for a lower degree of Hh oligomerization on the cell surface. A Shh–C4S complex as observed in the crystal structure is framed within a dashed box.