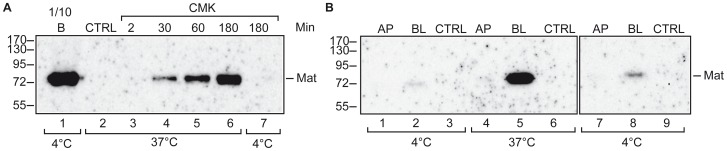
Figure 2. Biotin-RRQR-CMK reacts with a subset of matriptase molecules on the surface of Caco-2 cells.
(A) Eleven days post-confluent Caco-2 cells grown on Transwell filters were labeled with 50 µM biotin-RQRR-CMK from the basolateral side for the times indicated (2–180 min) at 37°C (lanes 3–6) or for 180 min at 4°C (lane 7). As a measure of the steady state level of matriptase, membrane proteins on the basolateral plasma membrane of filter-grown Caco-2 cells were labeled by incubation with S-NHS-SS-biotin at 4°C for 30 min (lane 1). As a negative control, cells were labeled from the basolateral side with 50 µM control peptide; biotin-RQRR (lane 2). All cells were lysed and biotinylated proteins were precipitated using streptavidin-coated resin and were analyzed by non-reducing SDS-PAGE and Western blotting using the monoclonal matriptase antibody; M32. A tenth of the surface biotinylated sample was loaded (lane 1); whereas total sample volume was loaded for the other samples (lanes 2-7). (B) Caco-2 cells grown on Transwell filters were labeled with the biotin-RQRR-CMK peptide inhibitor from either the apical (lanes 1, 4, and 7) or the basolateral (lanes 2, 5, and 8) side for 180 min at either 4°C or 37°C. An overexposure of lanes 1–3 is shown in lanes 7–9. As a negative control, cells were labeled from the basolateral side with a peptide corresponding to the inhibitory peptide but lacking the CMK moiety (CTRL, lanes 3, 6, and 9). Cells were lysed and the lysates of multiple filters were pooled. Biotinylated proteins were precipitated using streptavidin-coated resin and the streptavidin pull downs were released by boiling in SDS-PAGE samples buffer and analyzed by SDS-PAGE and Western blotting using the monoclonal M32 antibody. Positions of the molecular weight markers (kDa) are indicated on the left. Results shown are representative of 3 independent experiments.