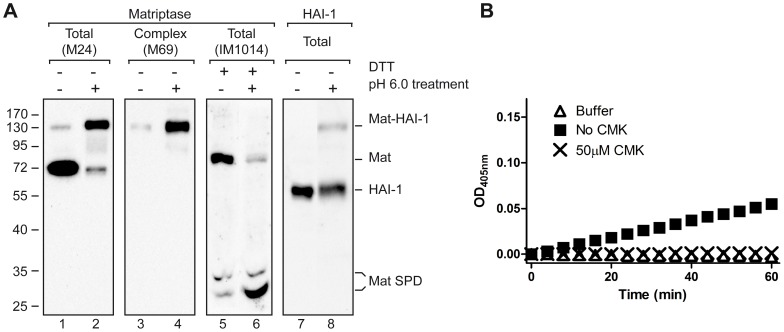
Figure 3. Arg614-cleaved matriptase is able to form complexes with HAI-1 and biotin-RQRR-CMK at pH 6.0.
(A) Eleven days post-confluent filter-grown Caco-2 cells were treated with either a physiologically phosphate buffer pH 6.0 for 20 min (lanes 2, 4, 6, and 8) from both the apical and the basolateral side or left untreated (lanes 1, 3, 5, and 7). Cells were lysed and lysates were analyzed by Western blotting using antibodies against total matriptase (M24; lanes 1 and 2), matriptase SPD (IM1014; lanes 5 and 6), matriptase-HAI-1 complex (M69; lanes 3 and 4) and HAI-1 (lanes 7 and 8). Samples in lanes 1–4, 7, and 8 were not boiled to avoid dissociation of matriptase-HAI-1 complexes, while samples in lanes 5 and 6 were boiled and reduced to dissociate the S-S bridged SPD from the stem domain of activated matriptase in order to distinguish between the SEA domain-cleaved form (70 kDa) and the Arg614 cleaved form (25–30 kDa). Treatment with phosphate buffer pH 6.0 and DTT is indicated by +/−. Positions of the molecular weight markers (kDa) are indicated on the left. (B) A solution of 0.2 µM SPD was incubated for 10 min at 37°C with (crosses) or without (squares) 50 µM biotin-RQRR-CMK before addition the chromogenic substrate to a final concentration of 300 µM. All experiments were performed in 20 mM citric acid buffer pH 6.0, 140 mM NaCl and 0.1% BSA at 37°C. Results shown are representative of 3 independent experiments.