FIGURE 2.
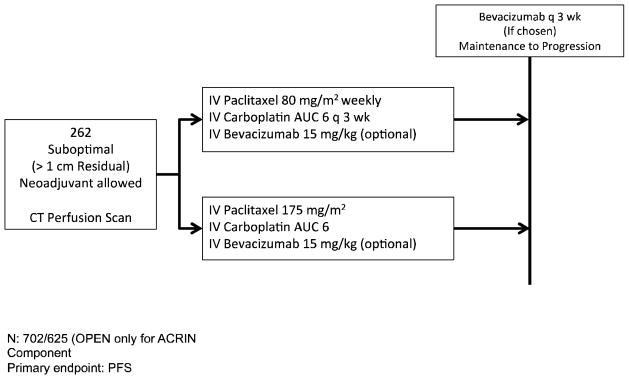
Schema for GOG 262. This trial was initially open for patients with suboptimal disease (> 1cm postoperative tumor residuum). Major amendments were enacted to allow patients undergoing neoadjuvant chemotherapy followed by surgery, and upon the enrollment completion of GOG 252, patients with optimal tumor cytoreduction. The trial has met its accrual target but is still enrolling patients who are participating on the perfusion imaging sub-study (ACRIN 6695). This trial, as opposed to GOG 252, 218, ICON7 and OCTAVIA allows for bevacizumab to be administered until progression or unacceptable toxicity in both arms (See text).
