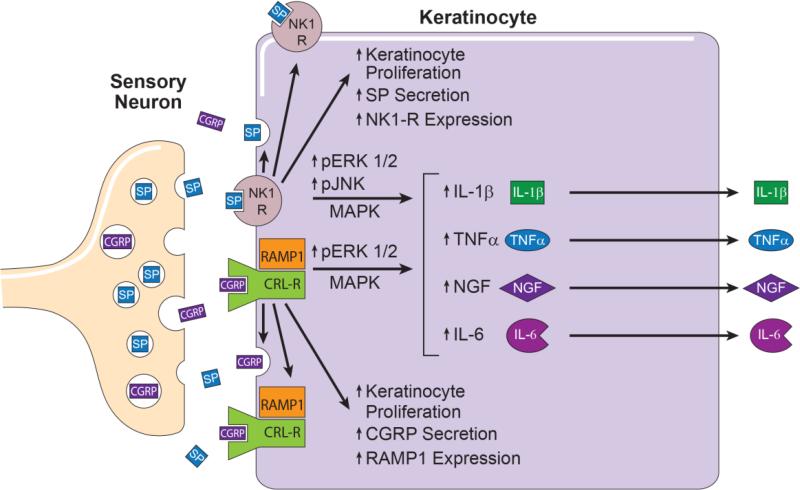
Figure 12. This schematic summarizes the results of these experiments.
Cutaneous primary sensory afferents release SP and CGRP in the epidermis. These neuropeptides then diffuse through the interstitial space to bind and activate their cognate receptors on the keratinocyte cell surface, the SP NK1 receptor and the CGRP receptor dimer complex of CRLR and RAMP1. Keratinocyte NK1 receptor activation stimulates cellular proliferation, SP expression and secretion, NK1 receptor expression, and the phosphorylation and activation of ERK 1/2 and JNK MAPK intracellular transcription factors that stimulate TNFα, IL-1β, IL-6, and NGF expression and secretion. Similarly, activation of the keratinocyte CGRP receptor dimer complex stimulates keratinocyte proliferation, CGRP expression and secretion, RAMP1 receptor expression, and the phosphorylation and activation of ERK 1/2 MAPK, an intracellular transcription factor that up-regulates TNFα, IL-6, and NGF expression and secretion. Keratinocyte secreted inflammatory cytokines and NGF can directly activate their cognate receptors expressed on cutaneous sensory afferent neurons, with subsequent pain sensitization. Keratinocyte secreted inflammatory mediators also can activate various cellular components of the innate and adaptive immune systems, thus supporting the development of inflammatory skin diseases and nociceptive sensitization. CGRP, calcitonin gene-related peptide; SP, substance P; NK1-R, neurokinin 1 receptor; CRL-R, calcitonin receptor –like receptor; RAMP1 receptor activity-modifying protein; MAPK, mitogen activated protein kinases; ERK1/2; extracellular signal related kinases 1/2; JNK, c-Jun N-terminal kinases; IL, interleukin; TNF α, tumor necrosis factor α; NGF, nerve growth factor.