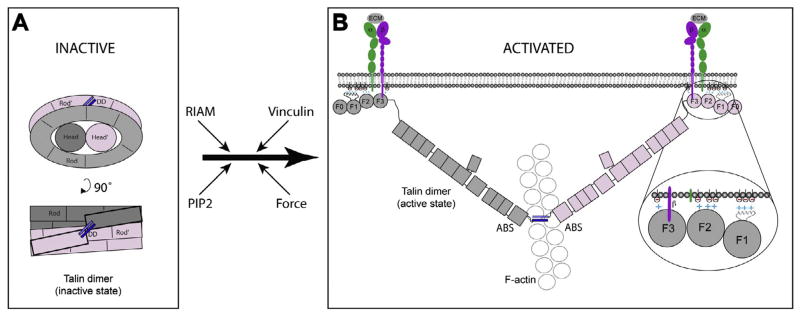
Fig. 8.
Schematic diagram of inactive and activated talin. (A) Compact inactive form of the talin dimer. The talin rod forms a donut-shaped structure with the talin heads occupying the hole in the center. The talin subunits are colored pink and grey and the dimerization helix is in blue. (B) When activated, the talin dimer adopts an extended structure in which the F3 FERM domains bind to the cytoplasmic domains of β-integrin subunits (purple), while basic residues on the F1, F2 and F3 FERM domains engage acidic membrane phospholipids (inset). Both interactions are required for integrin activation and binding to the extracellular matrix (ECM) (Anthis et al., 2009). The C-terminal actin-binding site (as a dimer) binds to a single actin filament (Gingras et al., 2008). Possible pathways to talin activation are indicated. Force exerted on talin is thought to facilitate vinculin binding to the talin rod, stabilizing integrin/talin/actin complexes, but this is not shown in the diagram.