Abstract
Aim
To retrospectively investigate the changes of SpO2 and respiratory drive in preterm infants at birth after administration of 100% oxygen.
Methods
Respiratory parameters, FiO2 and oximetry of infants <32 weeks gestation before and after receiving FiO2 1.0 were reviewed during continuous positive airway pressure (CPAP) or positive pressure ventilation (PPV).
Results
Results are given as median (IQR) or percentages where appropriate. Suitable recordings were made in 50 infants (GA 27 (26–29) weeks), 17 received CPAP and 33 PPV. SpO2 increased rapidly in the first minute after FiO2 1.0 and remained stable. The duration of FiO2 1.0 tended to be shorter in the CPAP group than in the PPV group (CPAP vs. PPV: 65 (33–105) vs. 100 (40–280) s; p = 0.05), SpO2 >95% occurred more often in PPV group (53% vs. 69%) and lasted longer (70(40–95) vs. 120(50–202) s). In CPAP group, minute volume increased from 134 (76–265) mL/kg/min 1 minute before to 240 (157–370) mL/kg/min (p<0.01) 1 minute after start FiO2 1.0 and remained stable at 2 minutes (252 (135–376) mL/kg/min; ns). The rate of rise to maximum tidal volume increased (from 13.8 (8.0–22.4) mL/kg/s to 18.2 (11.0–27.5) mL/kg/s; p<0.0001) to 18.8 (11.8–27.8) mL/kg/s; ns). In the PPV group respiratory rate increased from 0(0–4) to 9(0–20) at 1 minute (p<0.001) to 23 (0–34) breaths per minute at 2 minutes (p<0.01).
Conclusion
In preterm infants at birth, a rapid increase in oxygenation, resulting from a transient increase to 100% oxygen might improve respiratory drive, but increases the risk for hyperoxia.
Introduction
Hyperoxemia may lead to hyperoxia causing oxidative stress and tissue injury which should be avoided in infants at birth [1], [2]. Meta-analyses indicate that resuscitation of term infants at birth with air significantly reduced mortality compared with those resuscitated with fraction of inspired oxygen (FiO2) of 1.0 [1]–[6]. International resuscitation guidelines now recommend term infants should start in air [2], [7], [8]. Less clinical data are available for preterm infants, but guidelines now recommend to use oxygen judiciously during stabilization of preterm infants at birth [2], [7]–[9].
Since SpO2 percentiles were introduced [10] lower SpO2-targets in the first minutes after birth are accepted. However, hypoxia inhibits breathing movements in the fetus [11]. Although O2 sensitivity of infants changes in days-weeks after birth [12] and most preterm infants breathe at birth [13], [14], it is not known when the hypoxia-mediated switch from respiratory suppression to stimulation occurs. Possibly hypoxia immediately after birth will produce a weakened or absent respiratory drive as shown in preterm lambs [12]. In contrast, it has been shown in asphyxiated term infants [15] and animals [16] that applying 100% oxygen with no titration delayed the time of the first breath.
From 2008 until 2010, the local guidelines of the Royal Women's Hospital (Melbourne, Australia) and the Leiden University Medical Center (Leiden, the Netherlands) recommended starting in air and switching to FiO2 1.0 if needed and then titrating down in preterm infants at birth. An oxygen saturation (SpO2) ≤70% at 5 minutes was used to increase FiO2 [10]. The immediate switch to 100% was a pragmatic choice, but immediate FiO2 reduction was advocated once the infant was stabilized.
Our aim was to investigate the change in SpO2 and respiratory drive in preterm infants right after birth in the delivery room after switching from air to FiO2 1.0.
Methods
The local institutional review boards (IRBs) of the Leiden University Medical Center (Commissie Medische Ethiek, Leids Universitair Medisch Centrum) and Royal Women's Hospital (the Human Research Ethics Committee, Royal Woman's Hospital) approved physiological- and video recordings at birth in the delivery room when respiratory support was necessary for research purposes. Written parental consent to use the recordings for research was obtained after birth. A retrospective study was performed in both hospitals with data collected between 2008 and 2010. During the period of data collection local guidelines recommended that support was started with air and switched to FiO2 1.0 when: 1) cardiac massage was needed, 2) positive pressure ventilation (PPV) was administered for 1 minute and heart rate (HR) was <100 beats per minute (bpm) or 3) SpO2 <70% at 5 minutes. FiO2 was then titrated down as quickly as possible (when SpO2 >90%). Recordings were only made when the research team was available.
Respiratory support was delivered with a T-piece resuscitator (Neopuff, Fisher & Paykel, Wellington, New Zealand) and face mask. Local resuscitation guidelines recommended to start PPV (20–25/5 cmH2O) in preterm infants during apnea or HR<100 bpm. In breathing infants and HR>100 bpm, CPAP (5–6 cmH2O) is given. Changing pressures was left to the discretion of the caregiver.
The use of a respiratory monitor (Acutronic Medical Systems AG, Hirzel, Switzerland), a Masimo SET pulse oximeter (Masimo Radical, Masimo Corporation, Irvine CA, USA), an oxylog (Teledyne, Poway CA, USA) and Spectra program (Spectra, Grove Medical Limited, Hampton, UK) for physiological recordings has been described in detail in previous publications [13].
All recordings of infants born at <32 weeks gestation between 2008 and 2010 were reviewed. Using video and respiratory function monitoring other interventions were identified performed during the analyzed period. Infants receiving FiO2 1.0 were identified and divided into two groups. This was based on the type of respiratory support they received around the time point FiO2 1.0 was started: group 1) infants were breathing on continuous positive airway pressure (CPAP) and group 2) received positive pressure ventilation (PPV).
In all infants we recorded when FiO2 was increased to 1.0, for what reason(s) (e.g. low HR, low SpO2), duration and the downward titration rate of FiO2 1.0. Furthermore we noted the increase in SpO2 duration of SpO2 >95%. We used SpO2 >95% as an indication for increased risk for hyperoxia.
In group 1 (CPAP group) the effect of FiO2 1.0 on respiratory drive was investigated. To measure the change in respiratory effort, we analyzed the respiratory rate (RR), expired tidal volume (Vte), minute volume (MV) and the rate of rise to maximum tidal volume (mL/kg/second) during inspiration from 1 minute before until two minutes after starting FiO2 1.0 (which served as a control period). To measure the maximum rate of tidal volume increase we used spontaneous breaths without mask leak (Vti = Vte).
In group 2 (PPV group) the tidal volumes and rate of rise will be influenced by the positive pressure ventilation given and we only analyzed RR of the spontaneous breaths from 1 minute before until two minutes after starting FiO2 1.0. Breaths in between and coinciding with inflations were identified according to previous described methods [15]. In apneic infants, starting time of breathing was noted.
As changing of FiO2 can influence flow and volume measurements [17]–[19], the respiratory monitor was tested in vitro by delivering a constant tidal volume using a glass syringe and different gas conditions. The results were used to give the following corrections: at FiO2 1.0, both inspired and expired tidal volumes were corrected by −6% when using cold dry gas and by −10% when heated gas was used.
Data are presented as mean (± SD) or median (IQR) where appropriate. Differences were analyzed with a paired samples t-test for parametric data or a Wilcoxon signed rank test for non-parametric data where appropriate using (SPSS for Windows, version 17.0.0, Chicago, IL, USA). A two-sided p-value <0.05 was considered statistically significant.
Results
Data from 80 recorded infants were reviewed, 30 were excluded (no respiratory support (n = 10), no supplemental oxygen (n = 7), low quality recordings (n = 12) and 1 infant was born dead). Thus, 50 infants with GA 27 (26–29) weeks were analyzed (table 1); during the study window (1 minute before–2 minutes after start of oxygen) 17 breathed on CPAP (CPAP-group) and 33 received PPV (PPV-group).
Table 1. Baseline characteristics for preterm infants breathing on CPAP and infants receiving positive pressure ventilation when a FiO2 of 1.0 was started.
| Characteristics | breathing on CPAP | Positive pressure ventilation | p-value |
| N = 17 | N = 33 | ||
| Gestational age, wk, mean (SD) | 28.9 (1.5) | 27.1 (2.1) | <0.01 |
| Birth weight, g, mean (SD) | 1073 (227) | 993 (311) | <0.0001 |
| Male Sex (%) | 10 (60) | 16 (49) | ns |
| Caesarean (%) | 8 (47) | 18 (54) | ns |
| Apgar at 1 min, median (IQR) | 6 (5–7) | 4 (2–6) | <0.05 |
| Apgar at 5 min, median (IQR) | 8 (8–8) | 7 (6–8) | <0.05 |
The infants in the CPAP-group did not receive PPV during or after the study window. In both groups ventilation pressures were not increased during the study window. (pressures given: CPAP-group CPAP level 5.3 (4.6–5.9) cmH2O, PPV-group; PIP 21.6 (20.3–24.9) cmH2O and PEEP 4.2 (3.5–4.7) cmH2O) and no readjustments of mask position were observed. After the study window 1 infant of the CPAP-group was intubated, but reason was unclear. In the PPV-group, 5 infants were intubated after the study window for apnea and low SpO2 despite FiO2 1.0. Cardiac massage was not provided to any infant.
Supplemental oxygen
In the CPAP-group FiO2 1.0 was started 300 (225–315) seconds after birth and was given for 65 (33–105) seconds. FiO2 was weaned in 20 (5–60) seconds to 21 (21–21) %. In all infants oxygen was started for low SpO2 and HR was >100 bpm.
In the PPV-group FiO2 1.0 was started 180 (120–270) seconds after birth and was given for 100 (40–280) seconds. FiO2 was weaned in 25 (10–47) seconds to 21 (21–30)%.
Oxygen saturation
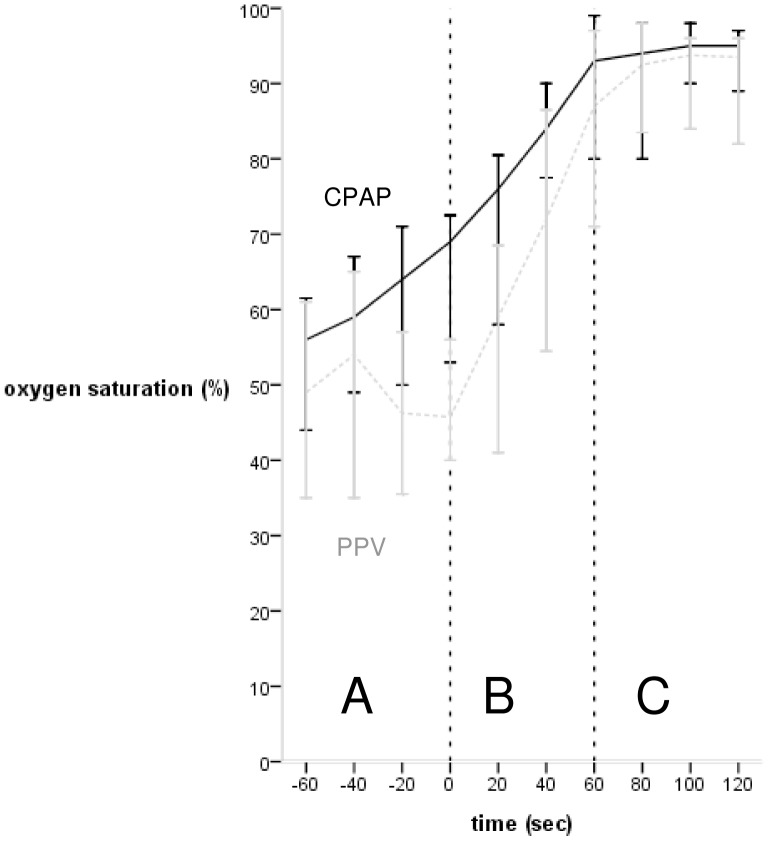
In all patients the fastest increase in SpO2 occurred in the first minute after starting oxygen (figure 1) (CPAP-group: from 62 (16)% to 87 (12)% at 1 minute after and to 93 (5)% at 2 minutes after starting oxygen, PPV-group: from 45 (19)% to 80 (24)% (p<0.001) after 1 minute and to 87 (19)% after 2 minutes (figure 1).
Figure 1. Oxygen saturation (%) of infants on CPAP and infants needing PPV in the minute before and 2 minutes after start of FiO2 1.0.
Black = CPAP-group, light grey = PPV-group, (A) minute before start FiO2 1.0, (B) first minute after start FiO2 1.0, (C) second minute after start FiO2 1.0.
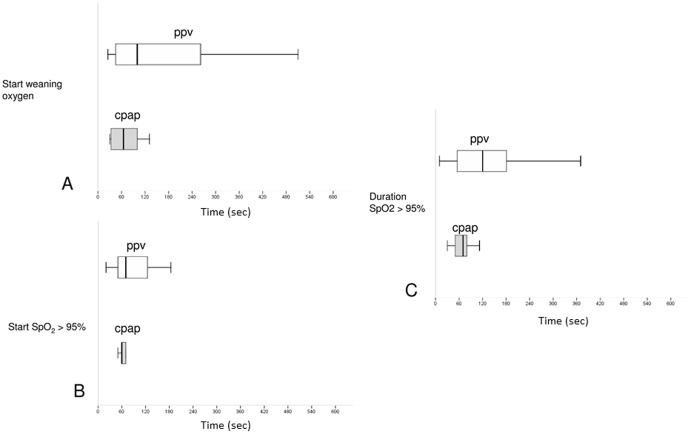
SpO2 >95% occurred in 9/17 (53%) infants of in group 1 and in 23/33 (69%) infants of group 2. The starting point and duration of SpO2 >95% are depicted in figure 2
Figure 2. Box plots showing median (IQR) starting time of weaning FiO2 1.0 (A), starting time of SpO2 >95%, (B) in seconds after FiO2 1.0 is started and duration of SpO2 >95%, (C) in infants on CPAP and infants needing PPV. Grey = CPAP-group, white = PPV-group.
Changes on respiratory drive when breathing on CPAP
In the CPAP-group, increasing FiO2 to 1.0 increased RR from 30 (18–41) 1 min before to 35 (24–45) breaths per minute (ns) 1 minute after to 39 (31–44) breaths per minute (p<0.05) in the 2nd minute.
Vte and MV increased significantly in 1st minute after increasing FiO2 to 1.0 and remained stable in the 2nd minute (Vte: from 4.9 (2.3–8.8) mL/kg to 6.7 (3.6–10.4) mL/kg (p<0.001) to 6.5 (3.7–10.2) mL/kg (ns); MV: from 134 (76–265) mL/kg/min to 240 (157–370) mL/kg/min (p<0.01) to 252 (135–376) mL/kg/min (ns)).
The rate of rise to maximum tidal volume increased from 13.8 (8.0–22.4) mL/kg/s in the minute before to 18.2 (11.0–27.5) mL/kg/s (p<0.0001) in the minute after increasing FiO2 to 1.0 and remained stable at 18.8 (11.8–27.8) mL/kg/s (ns) in the 2nd minute.
Changes on respiratory drive when receiving PPV
PPV was given in 23 apneic infants and in 10 infants for poor respiratory drive. Apneic infants started breathing 80 (50–180)s after FiO2 1.0 and at that moment SpO2 was 87% (11) and HR 147 (19) bpm.
RR increased from 0 (0–4) 1 min before to 9 (0–20) breaths per minute (p<0.001) 1 minute after to 23 (0–34) breaths per minute (p<0.01) in the 2nd minute after switching to FiO2 1.0.
Discussion
We investigated the influence of switching from air to FiO2 1.0 on SpO2 and respiratory drive in preterm infants at birth. Most infants with SpO2 near the 10th percentile had a good HR but FiO2 was increased to 1.0 because of low SpO2. After increasing FiO2 to 1.0, respiratory drive improved simultaneously with a rapid increase in SpO2. However, SpO2 >95% occurred in the majority of infants, especially in the infants who received PPV, which probably reflects the difficulty of simultaneously performing PPV and titrating oxygen. These observations suggest that targeting a higher percentile as currently recommended in international guidelines [7], [8] (25th–50th percentile) might improve respiratory drive. A more stepwise increase in FiO2 and more diligence in reducing FiO2, for example when SpO2 >85%, could reduce the risk of hyperoxia.
We observed that preterm infants started to breathe more vigorously, as indicated by an increased rate and effort, once FiO2 was increased and SpO2 improved. Antenatally, hypoxia suppresses fetal breathing movements [11] whereas postnatally hypoxia stimulates breathing. The sensitivity increases during days-weeks after birth [12]. However, the mechanisms driving the large inspiratory efforts and controlling the switch to continuous breathing after birth are unknown, although increasing arterial PO2 may be involved [20]. We speculate that infants in our study, who failed resuscitation with air, respiratory support was insufficient to aerate the lung and supplemental oxygen was required to compensate. We suggest that the resulting increase in oxygenation increased drive from the respiratory center and respiratory effort, which increased lung aeration and FRC. This would explain why FiO2 1.0 was only required for a short time and could be rapidly weaned allowing most infants to remain stable with little extra oxygen. Although our weaning rate was fast, studies comparing high versus moderate FiO2 levels in preterm infants found similar levels of FiO2 at 10 minutes [9], [21]–[23].
Experimental studies have shown that pulmonary vascular resistance at birth is related to ventilation onset and oxygen had little impact [24]–[26]. Also, Sobotka et al. found that increasing FiO2 to 1.0 in hypoxic lambs just after birth improved blood oxygenation, but had no effect on lung compliance and pulmonary blood flow [25]. This supports the hypothesis that increased oxygenation after FiO2 1.0 is achieved by increasing the partial pressure gradient for oxygen diffusion compensating the ventilation perfusion mismatch due to low FRC [25].
The reported Vte increased in infants on CPAP could be explained by improved lung compliance. However, volume increase occurred right after increasing FiO2 and remained stable in the minute thereafter. Also, RR increase cannot be explained by improving compliance. Alternatively, increased pressures could have elevated Vte, but these remained unchanged. Increasing FiO2 increases gas density which can influence measurements [17]–[19]. However, after correction, tidal volumes remained significantly larger after FiO2 1.0 and when considering the rate of rise is also increased it is more likely to be the infant's own effort.
Although studies showed it is feasible to support preterm infants at birth with a FiO2 of <1.0, most infants starting with low FiO2 levels needed an increased FiO2 (0.45–0.6) to reach target SpO2 levels [21], [22]. However, the different approaches make it difficult to compare these studies with our observational data reported in this study. We often observed SpO2 below target. Therefore, starting in air may not be the right approach. Although it is unclear how detrimental a short period of FiO2 1.0 is at birth (1–2 minutes), more vigilance in preventing SpO2 levels >95% is needed [2].
In line with our recent report [13], we observed that oxygen use was not always according to the guidelines. Oxygen was given earlier or later than recommended. In the PPV-group a SpO2 >95% occurred more often and lasted longer, increasing the chances of hyperoxia. These observations may indicate the algorithm was difficult to follow. This will become even more difficult if separate SpO2 targets for each minute after birth are defined and may lead to a change in focus away from adequate ventilation. Adding an extra person to the resuscitation team could be helpful.
Limitations
The retrospective nature of the study and the relative small sample size precludes any hard conclusion regarding respiratory drive and oxygenation. Recording respiratory parameters at birth is challenging, similar studies do not include large number of infants [27]–[31]. The infants included in this study is a sample of the preterm infants born in the hospitals and a selection bias could have occurred. However, the sample was randomly chosen as recordings were performed if the research team was available. The observed variation in starting time of FiO2 1.0 complicates comparing with the respiratory drive of infants receiving air. Also, the observational nature of this study prevented us to have a control group of infants needing no support. However, we were interested in the effect of FiO2 1.0 and measurements before starting oxygen served as a control period.
Conclusions
We observed that during respiratory support of preterm infants switching from air to FiO2 1.0 increased the risk for hyperoxia. No hard conclusions can be drawn, but our observations might suggest that respiratory drive increased after supplemental oxygen was given and oxygenation improved. The role of SpO2 levels in stimulating respiratory drive at birth merits further investigation.
Funding Statement
A.B. te Pas is the recipient of a Veni-grant, The Netherlands Organisation for Health Research and Development (ZonMw), part of the Innovational Research Incentives Scheme Veni-Vidi-Vici, project number 91612027. J.J. van Vonderen is the recipient of a Willem-Alexander Children's Foundation scholarship www.willem-alexanderkinderfonds.nl. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Higgins RD, Bancalari E, Willinger M, Raju TN (2007) Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics 119 (4) 790–6. [DOI] [PubMed] [Google Scholar]
- 2. Vento M, Saugstad OD (2011) Oxygen supplementation in the delivery room: updated information. J Pediatr 158 (2 Suppl) 5–7. [DOI] [PubMed] [Google Scholar]
- 3. Hellstrom-Westas L, Forsblad K, Sjors G, Saugstad OD, Björklund LJ, et al. (2006) Earlier Apgar score increase in severely depressed term infants cared for in Swedish level III units with 40% oxygen versus 100% oxygen resuscitation strategies: a population-based register study. Pediatrics 118 (6) 1798–1804. [DOI] [PubMed] [Google Scholar]
- 4. Rabi Y, Rabi D, Yee W (2007) Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation 72 (3) 353–363. [DOI] [PubMed] [Google Scholar]
- 5. Saugstad OD, Ramji S, Soll RF, Vento M (2008) Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 94 (3) 176–182. [DOI] [PubMed] [Google Scholar]
- 6. Tan A, Schulze A, O'Donnell CP, Davis PG (2005) Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev 2: CD002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, et al. (2010) Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 122 (16 Suppl 2) 516–538. [DOI] [PubMed] [Google Scholar]
- 8. Wyllie J, Perlman JM, Kattwinkel J, Atkins DL, Chameides L, et al. (2010) Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation (Suppl 1) 260–287. [DOI] [PubMed] [Google Scholar]
- 9. Vento M, Moro M, Escrig R, Arruza L, Villar G, et al. (2009) Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009 124 (3) 439–449. [DOI] [PubMed] [Google Scholar]
- 10. Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, et al. (2010) Defining the reference range for oxygen saturation for infants after birth. Pediatrics 125 (6) 1340–1347. [DOI] [PubMed] [Google Scholar]
- 11. Gluckman PD, Johnston BM (1987) Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol 382: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davey MG, Moss TJ, McCrabb GJ, Harding R (1996) Prematurity alters hypoxic and hypercapnic ventilatory responses in developing lambs. Respir Physiol 105 (1–2) 57–67. [DOI] [PubMed] [Google Scholar]
- 13. Schilleman K, Siew ML, Lopriore E, Morley CJ, Walther FJ, et al. (2012) Auditing resuscitation of preterm infants at birth by recording video and physiological parameters. Resuscitation 83 (9) 1135–1139. [DOI] [PubMed] [Google Scholar]
- 14. O'Donnell CP, Kamlin CO, Davis PG, Morley CJ (2010) Crying and breathing by extremely preterm infants immediately after birth. J Pediatr 156 (5) 846–47. [DOI] [PubMed] [Google Scholar]
- 15. Saugstad OD, Rootwelt T, Aalen O (1998) Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics 102 (1) e1. [DOI] [PubMed] [Google Scholar]
- 16. Bookatz GB, Mayer CA, Wilson CG, Vento M, Gelfand SL, et al. (2007) Effect of supplemental oxygen on reinitiation of breathing after neonatal resuscitation in rat pups. Pediatr Res 61 (6) 698–702. [DOI] [PubMed] [Google Scholar]
- 17. Fischer HS, Roehr CC, Proquitte H, Wauer RR, Schmalisch G (2008) Assessment of volume and leak measurements during CPAP using a neonatal lung model. Physiol Meas 29 (1) 95–107. [DOI] [PubMed] [Google Scholar]
- 18. Foitzik B, Schmalisch G, Wauer RR (1994) Effect of physical properties of respiratory gas on pneumotachographic measurement of ventilation in newborn infants. Biomed Tech (Berl) 39 (4) 85–92. [DOI] [PubMed] [Google Scholar]
- 19. Roske K, Foitzik B, Wauer RR, Schmalisch G (1998) Accuracy of volume measurements in mechanically ventilated newborns: a comparative study of commercial devices. J Clin Monit Comput 14 (6) 413–420. [DOI] [PubMed] [Google Scholar]
- 20. Givan DC (2003) Physiology of breathing and related pathological processes in infants. Semin Pediatr Neurol 10 (4) 271–280. [DOI] [PubMed] [Google Scholar]
- 21. Escrig R, Arruza L, Izquierdo I, Villar G, Sáenz P, et al. (2008) Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics 121 (5) 875–81. [DOI] [PubMed] [Google Scholar]
- 22. Rabi Y, Singhal N, Nettel-Aguirre A (2011) Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR study. Pediatrics 128 (2) e374–e381. [DOI] [PubMed] [Google Scholar]
- 23. Wang CL, Anderson C, Leone TA, Rich W, Govindaswami B, et al. (2008) Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics 121 (6) 1083–9. [DOI] [PubMed] [Google Scholar]
- 24. Lakshminrusimha S, Steinhorn RH, Wedgwood S, Savorgnan F, Nair J, et al. (2011) Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J Appl Physiol 111 (5) 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobotka KS, Hooper SB, Allison BJ, Te Pas AB, Davis PG, et al. (2011) An initial sustained inflation improves the respiratory and cardiovascular transition at birth in preterm lambs. Pediatr Res 70 (1) 56–60. [DOI] [PubMed] [Google Scholar]
- 26. Teitel DF, Iwamoto HS, Rudolph AM (1990) Changes in the pulmonary circulation during birth-related events. Pediatr Res 27 (4 Pt 1) 372–378. [DOI] [PubMed] [Google Scholar]
- 27. Te Pas AB, Kamlin CO, Dawson JA, O'Donnell C, Sokol J, et al. (2009) Ventilation and spontaneous breathing at birth of infants with congenital diaphragmatic hernia. J Pediatr 154 (3) 369–373. [DOI] [PubMed] [Google Scholar]
- 28. Schmolzer GM, Dawson JA, Kamlin CO, O'Donnell CP, Morley CJ, et al. (2011) Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 96 (4) 254–257. [DOI] [PubMed] [Google Scholar]
- 29. Te Pas AB, Davis PG, Kamlin CO, Dawson J, O'Donnell CP, et al. (2008) Spontaneous breathing patterns of very preterm infants treated with continuous positive airway pressure at birth. Pediatr Res 64 (3) 281–285. [DOI] [PubMed] [Google Scholar]
- 30. Finer NN, Rich W, Wang C, Leone T (2009) Airway obstruction during mask ventilation of very low birth weight infants during neonatal resuscitation. Pediatrics 123 (3) 865–869. [DOI] [PubMed] [Google Scholar]
- 31. Te Pas AB, Wong C, Kamlin CO, Dawson JA, Morley CJ, et al. (2009) Breathing patterns in preterm and term infants immediately after birth. Pediatr Res 65 (3) 352–356. [DOI] [PubMed] [Google Scholar]