Abstract
Because α-synuclein (Snca) is involved in neuroinflammatory response, we determined if its expression altered blood-brain barrier (BBB) permeability. To induce increased BBB permeability, Snca gene-ablated (KO) and wild-type (WT) mice were injected (i.p.) with lipopolysaccharide (LPS). To assess changes in BBB permeability, Evans blue was injected (i.p.) and extravasation into the brain assessed using fluorescence spectroscopy. WT mice had a significant increase in BBB permeability at 1, 3, and 6 hours post-injection of LPS relative to untreated mice. Contrary to WT mice, LPS did not induce a time-dependent change in BBB permeability in KO mice. Although brain edema is associated with increased BBB permeability, no significant difference in edema was found between groups. These results show that Snca expression is associated with increased reactive opening of the BBB in response to LPS.
Keywords: Snca, α-synuclein, blood-brain barrier, edema, lipopolysaccharide
Introduction
α-Synuclein (Snca) is a small protein composed of 140 amino acids and is highly conserved across vertebrates. While its definitive functions are still being elucidated, Snca has been implicated in a wide range of functions including: brain fatty acid metabolism [8;11;14;21;23;24;47], synaptic vesicle mobilization [10], dopamine biosynthesis [38], dopamine transporter modulation [53], chaperone activity [3], and regulation of inflammatory response [5;6;22;44]. Mirroring its wide functionality, Snca is widely expressed amongst cell types in the central nervous system (CNS) including astrocytes, neurons, oligodendroglia, and microglia [5;11;33;36].
Microglia from Snca−/− (KO) mice have a higher basal reactive state than those from wild-type (WT) mice, suggesting that Snca has a role in modulating neuroinflammation [5]. Microglia lacking Snca have an exaggerated inflammatory response to lipopolysaccharide (LPS, 25 ng/ml), including elevated TNFα and IL-6 secretion [5] and elevated prostaglandin (PG) biosynthesis [6]. This is consistent with our observations that brain PG levels are elevated following global ischemia in the KO mice [22]. Because Snca is required for normal rates of arachidonate incorporation and recycling into brain phospholipids [23], the elevated levels of PG are likely due to a reduction in the recycling of released arachidonic acid in the KO mice, thereby increasing the amount of substrate for downstream PG synthesis [20]. As PG are important in blood brain-brain barrier function (BBB) [1;4;27], the question arises whether Snca expression might impact BBB function.
The BBB is a physical and metabolic barrier that protects the central nervous system from harmful agents in the blood as well as maintaining a homeostatic environment for the brain [2;16;32;39]. Inflammation of the BBB leads to an undesirable increase in permeability of the barrier and is associated with neurological disease [49]. Many signaling molecules can induce increased permeability of the BBB, including TNFα and other cytokines [1;7;28], while LPS induces increased BBB permeability via stimulating formation and release of cytokines and other molecules [42;55]. Because it is unknown if Snca expression alters BBB function, we examined the impact of LPS-induced BBB changes in Snca+/+ (WT) and Snca−/−(KO) mice.
Materials and Methods
Mice
This study was conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication 80–23) and under an animal protocol approved by the IACUC at the University of North Dakota (Protocol 0708-1c). Mice used were the 129/SvEv strain and the Snca gene-ablated mice were generated via gene-targeted deletion of Snca [10]. Male mice aged 8 to 13 months and between 27–43 g, were given standard laboratory chow and water ad libitum.
LPS Treatments
Two different doses and treatment regimens were used to determine whether a dose-based response was present in WT mice. LPS derived from E. coli 055:B5 J (Sigma cat# L2880) was dissolved in phosphate buffered saline (0.25 mg/ml) and injected intraperitoneally (i.p.) using two different treatment regimens. In an initial set of experiments, WT mice were either treated with divided doses of LPS (3 mg/kg per dose, i.p.) at 0, 6, and 24 h [35;56] or a single dose of LPS (1 mg/kg, i.p.) [9;52]. Mice receiving divided doses of LPS were injected with Evans blue (i.p.) 1 h after the final dose of LPS was administered and perfused 2 hours later. For the group receiving the single dose of LPS, mice were injected with Evans blue (i.p.) 1 h after the LPS was administered and perfused 2 h later. For both groups, BBB permeability was assessed 3 h after injection of LPS and 2 h post-Evans blue injection.
Because we found no difference in BBB permeability between the single dose and divided doses of LPS, we used a single dose of LPS (1 mg/kg, i.p.) to induce an inflammatory response in KO and WT mice and Evans blue dye extravasation was measured at either 0, 1, 3, or 6 hours post-LPS injection to determine the temporal impact of Snca expression on LPS-induced BBB permeability. Thus, in some mice, Evans blue was injected (i.p.) prior to the introduction of LPS in order to have a consistent time (2 h) of circulating Evans blue prior to perfusion. In preliminary experiments, no interaction was seen between LPS and Evans blue, thus injection (i.p.) of LPS following Evans blue injection (i.p.) did not alter the activity of LPS. In all experiments, untreated mice were injected with vehicle (PBS) prior to Evans Blue dye injection.
Evans Blue Dye Injection and Extraction
Evans blue dye (2% wt/vol, 4 ml/kg in PBS; Sigma cat# 206334) [7;51] was injected i.p. and allowed to circulate for 2 hours prior then the mice were anesthetized with pentobarbital (165 mg/kg), the chest wall opened to expose the heart, and 0.2 ml of blood collected from the heart to assess plasma dye levels. Plasma was separated from whole blood using centrifugation. The mice were then perfused with heparinized PBS via a 23 gauge needle in the left ventricle until a clear fluid was obtained from the right atrium. PBS without heparin was initially used [51], however heparinized PBS (0.1 mg/L) produced more consistent results in preliminary studies, thus we used heparinized PBS for all perfusions in this study. The brains were then removed, weighed, and extravasated Evans blue dye was extracted with 50% trichloroacetic acid (TCA) [51].
Briefly, one brain hemisphere was weighed and then homogenized in 1 ml of 50% TCA (wt/vol) and subjected to centrifugation at 5,000 x g for 20 min. The supernatant was then diluted 1:3 with ethanol and concentration of Evans blue measured using fluorescence spectroscopy.
Evans blue in the plasma was extracted by adding 35 ul of plasma to 0.315 ml 50% TCA (wt/vol), mixed by vortexing, subjected to centrifugation at 5,000 x g for 20 min., and then 50 ul of the supernatant diluted with 150 ul ethanol. This sample was then diluted with 1:3 TCA to EtOH for fluorescence spectroscopy.
Evans Blue Dye Measurement
A Varian Eclipse fluorescence spectrophotometer was used to measure Evans blue concentrations in the samples. Settings were as follows: λex 620 nm, λem 680 nm, excitation slit 5 mm, emission slit 5 mm, and the fluorescence intensity was monitored and averaged over 25s. The inner filter effect was negligible as sample absorbance was less than 0.070 OD as measured using a Beckman DU-640 spectrophotometer on all samples tested. Standards were prepared with Evans blue dye in the final solvent composition.
Edema Measurement
To assess brain edema, hemispheres were weighed immediately after perfusion, and dried for 24 h at 95°C and then weighed. The hemispheres were then reweighed at 48 h to ensure that the samples had reached a steady state [48]. The percent brain water content was calculated as the difference between wet weight and dry weight divided by wet weight [17;48]. Prior to obtaining this data, perfusion was found not to alter the brain water content in a pilot experiment.
Statistics
Pearson’s rank correlation analysis was used to assess correlation of plasma Evans blue dye concentrations with brain Evans blue dye concentrations. All other results were analyzed using a one-way ANOVA and Tukey’s post test, p<0.05.
Results
Standard Curve
The standard curves exhibited a good linear response over a broad range and a curve from 5–50 pg/ml was used to quantify brain Evans blue dye and 50–1000 pg/ml curved used for plasma.
Dose-response in WT Mice
Evans blue content in the brain was not significantly different between the multiple dosing group (3 x 3 mg/kg, 331 +/− 64 pg/g ww, n=4 ) and single dosing group (1 mg/kg, 291 +/− 58 pg/g ww, n=4), although both were significantly different from control (167 +/− 49 pg/g ww, n=4) as determined by one-way ANOVA coupled with Tukey’s post-test, p<0.05.
Correlation of Brain Dye Extravasation with Plasma Dye Concentration
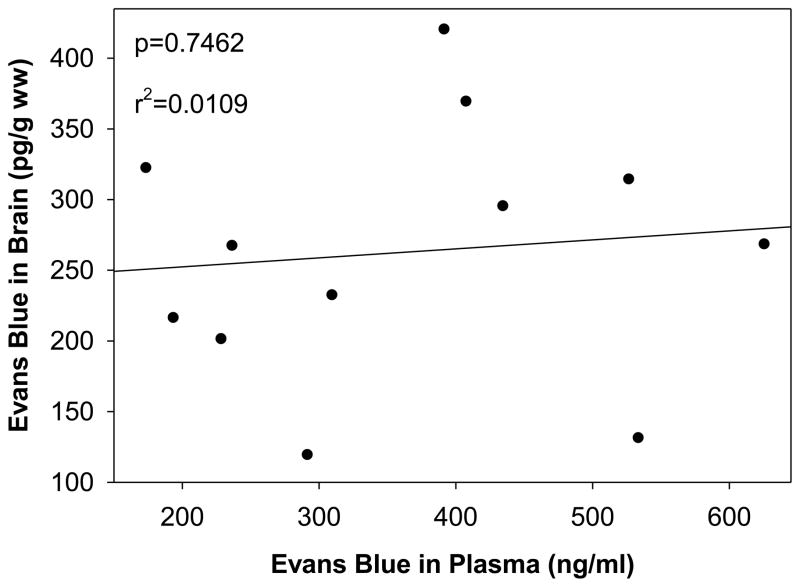
We found that the Evans blue dye extravasation from the plasma compartment into the brain was independent of plasma dye concentrations (Fig. 1), suggesting the process is saturated at the plasma dye concentrations studied.
Fig. 1.
Correlation of plasma Evans blue dye concentration with brain Evans blue dye concentration. Data analyzed using Pearson Rank correlation, p=0.7462
Impact of Snca Expression on the BBB Response to a Single Injection of LPS (1mg/kg)
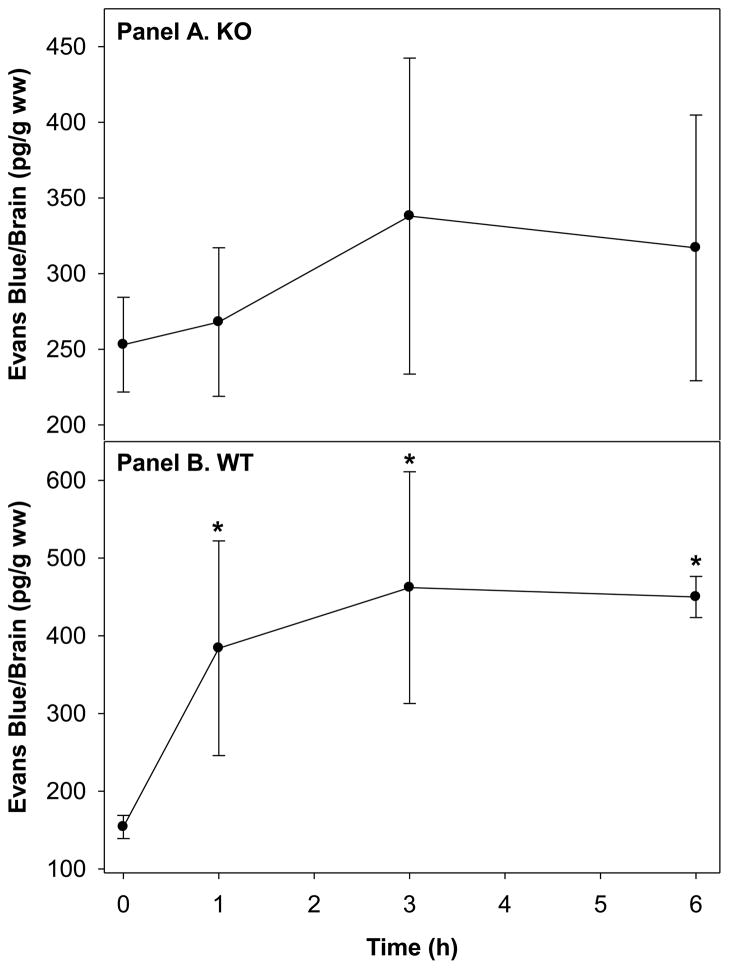
We found that the expression of Snca did not impact BBB permeability between groups in the absence of LPS (Fig 2, Panel A & B). There was no observed time-dependent effect on BBB opening in the KO mice (Fig. 2, Panel A). However, our results demonstrate a time-dependent increase in BBB opening in WT mice (Fig. 2, Panel B), suggesting the presence of Snca is important in this process. At 6 hour post-injection, there was a 3-fold increase in Evans blue extravasation into brains from WT mice as compared to T=0, indicating that the permeability of the BBB was indeed increased. This increased opening of the BBB was not accompanied by edema (Table 1), despite the 3-fold increase in Evans blue extravasation.
Fig. 2.
Effect of LPS treatment (1 mg/kg, i.p.) on Evans blue dye extravasation into the brain of brains of Snca −/− (KO, Panel A) and WT (Panel B) mice over time. The * indicates statistical significance from untreated WT mice, p<0.05 as determined using a one-way ANOVA and Tukey’s post-hoc test, n=3–5. There were no differences between groups.
Table 1.
Brain water content (% water) in WT and Snca KO brain following LPS injection.
| % Brain Water WT | % Brain Water KO | |||||
|---|---|---|---|---|---|---|
| Avg | SD | RSD | Avg | SD | RSD | |
| 0 h post-LPS | 77.6 | 0.6 | 0.7 | 78.0 | 0.2 | 0.2 |
| 1 h post-LPS | 77.5 | 0.3 | 0.3 | 77.7 | 0.2 | 0.2 |
| 3 h post-LPS | 77.4 | 0.2 | 0.3 | 77.5 | 0.3 | 0.4 |
| 6 h post-LPS | 77.4 | 0.3 | 0.4 | 77.8 | 0.4 | 0.5 |
Statistical analysis was done using a one-way ANOVA and a Tukey’s post-hoc test, n =4–5 for each group.
Discussion
There are several methods for inducing an inflammatory response in the brain using LPS. While delivery of LPS using an indwelling cannula in the fourth ventricle induces a change in arachidonic acid metabolism in a matter of days [45] and prolonged infusion over weeks results in cholinergic neuronal loss [43;54], this model requires an indwelling cannula and the inflammatory response occurs over time. The fragile nature of the KO mice precludes the subscapular insertion of the osmotic mini-pump for the duration used in these models. To circumvent this issue, we injected LPS via an intraperitoneal route to induce neuroinflammatory response [9;18;19;35;41;46;52;56]. In this model, doses can be given either as a single dose (1 mg/kg, i.p.) [9;52] or given in multiple doses (3 x 3 mg/kg) over 24 hours [35;56]. While we had expected the multiple dosing group to have a significant increase in Evans blue extravasation as compared to the single dosing regimen, this was not the case. In our hands, we found that multiple doses over 24 h resulted in the same opening of the BBB as the single dose given only 3 hours prior to killing the mice.
Evans blue dye is widely used to assess BBB permeability [7;13;30;51]. While some studies use a ratio of Evans blue dye in plasma to Evans blue dye in brain to quantify its extravasation into the brain [51], others only measure brain Evans blue dye levels to assess the extent of its extravasation [7;13;30]. While the ratio-metric method certainly has its advantages, we questioned the inherent variability of the intraperitoneal administration of Evans blue dye on plasma Evans blue dye levels. Correlating the plasma to brain concentrations of Evans blue, we found no correlation, indicating that in our model system, brain concentration is independent of plasma concentrations.
Our data demonstrate several key points. First, a single dose of LPS is sufficient to increase the permeability of the BBB and this occurs within 1 hour after exposure to LPS (i.p.) (Fig. 2B) in WT mice. This is important, because a single dose of LPS (5 mg/kg) results in a TNFα-induced profound chronic neuroinflammatory response, which leads to the progressive neurodegeneration of the substantia nigra beginning at 7 months post-injection [41]. Consistent with our study, others have demonstrated that the inflammatory response in the brain peaks at 1 h post-injection of LPS [41]. Interestingly, the KO mice did not demonstrate a time-dependent opening of the BBB barrier as assessed using Evan blue dye (Fig 2A). Although LPS treatment (i.p.) induces a response that is exacerbated by increasing age [26], the lack of a response in KO mice was not the result of an age difference with WT mice as all of the mice used in this study were between 8–13 months of age and age-matched within the study.
The 2.5 to 3-fold increase in BBB permeability is consistent with that reported in the literature [34;41;56], which is likely dependent upon on a vasodilatory PG-mediated process as permeability is reduced in the presence of indomethacin [56]. Because Snca ablation alters brain arachidonic acid metabolism [23] and increases PG formation [6;22], it increases the formation of both vasodilatory and vasoconstrictive PG. Thus although it might be anticipated that BBB opening during LPS (i.p.) challenge would exacerbate in the Snca gene-ablated mice, this was not the case. Rather increased BBB permeability was dependent upon the presence of Snca.
One potential explanation is the observed increase in in basal levels of β1 integrin in microglia from KO mice [5]. A similar increase in the level of β1 integrin on the endothelial cells and on astrocytes comprising the BBB may occur in the absence of Snca. Integrins have an important role in maintaining BBB integrity, including β1 integrin [12;50]. Importantly, β1 integrin is expressed on cerebral microvascular endothelial cells and on astrocytes in murine [31], in human [37], and in non-human primate [25;50] brain. In addition, β1 integrin is an important endothelial cell survival factor [29] and is a critical component of the BBB that is rapidly loss following focal brain ischemia [50]. Hence, β1 integrin is a considered a critical component of the endothelial cells and astrocytes comprising the BBB and are important for maintaining the integrity of the BBB [12;50]. Our observed results may very well be the result of enhanced β1 integrin levels in astrocytes and endothelial cells in KO mice, consistent with what we have observed in microglia from Snca KO mice [5].
Another potential explanation is that although there is enhanced PG formation in the absence of Snca [6;22], there may be a shift in the homeostasis between vasoconstrictive PG as opposed to those involved in vasodilation. Although thromboxane A2 (TXA2) is vasoconstrictive in the brain as it is elsewhere [15], both prostaglandin F2α(PGF2α) and prostaglandin E2 (PGE2) are also vasoconstrictive in the brain [40;57]. Certainly our observed increase in TXA2, PGF2α, and PGE2 in LPS-stimulated microglia from KO mice [22] as well as increased formation of these same PG following ischemic insult in KO mice [6], suggest that there is a preponderance of vasoconstrictive PG formation in the absence of Snca. Hence, the enhanced PG formation observed in these mice in the absence of Snca may very well account for the lack of responsiveness in KO mice.
The primary question addressed in our study was the impact of Snca expression on BBB integrity. Because we and others have demonstrated that Snca has a role in microglial response to LPS [5;6;36;44] and that Snca deficient mice demonstrate reduced arachidonic acid recycling [23] as well as increased prostaglandin formation [22], this work is a natural extension of our previous efforts to determine the impact of Snca expression in brain inflammatory response. Nonetheless, the dependence of increased BBB permeability on Snca expression is herein reported for the first time and suggests that Snca has an important role in BBB permeability, which is consistent with its emerging role in brain inflammatory response.
Highlights.
A single dose or multiple doses of lipopolysaccharid (LPS) equally opened the blood brain barrier (BBB)
Evans blue dye extravasation from plasma compartment into the brain was independent of plasma dye concentration.
Expression of alpha-synuclein was necessary for increasing BBB permeability elicited by single injection (i.p.) of LPS
Acknowledgments
This work was supported by an NIH grant NS060141 to EJM and a UND SMHS REMS summer research fellowship to AJ.
Footnotes
Conflict of Interest Statement
The authors declare there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cellular and Molecular Neurobiology. 2000;20:131–147. doi: 10.1023/a:1007074420772. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of Disease. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Ahn M, Kim SB, Kang M, Ryu Y, Kim TD. Chaperone-like activities of α-synuclein: α-synuclein assists enzyme activities of esterases. Biochemical and Biophysical Research Communications. 2006;346:1142–1149. doi: 10.1016/j.bbrc.2006.05.213. [DOI] [PubMed] [Google Scholar]
- 4.Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1β regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 5.Austin SA, Floden AM, Murphy EJ, Combs CK. α-Synuclein expression modulates microglial activation phenotype. Journal of Neuroscience. 2006;26(4):10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin SA, Rojanathammanee L, Golovko MY, Murphy EJ, Combs CK. Lack of alpha-synuclein modulates microglial phenotype in vitro. Neurochemical Research. 2011;36:994–1004. doi: 10.1007/s11064-011-0439-9. [DOI] [PubMed] [Google Scholar]
- 7.Ay I, Francis JW, Jr, Brown RH. VEGF increases blood-brain barrier permeability to Evans blud dye and tetanus toxin fragment C but not adeno-associated virus in ALS mice. Brain Research. 2008;1234:198–205. doi: 10.1016/j.brainres.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 8.Barceló-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in α-synuclein gene-ablated mice. Journal of Neurochemistry. 2007;101:132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- 9.Blais V, Turrin NP, Rivest S. Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. Journal of Neurochemistry. 2005;95:1563–1574. doi: 10.1111/j.1471-4159.2005.03480.x. [DOI] [PubMed] [Google Scholar]
- 10.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KC, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. Journal of Neuroscience. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castagnet PI, Golovko MY, Barceló-Coblijn G, Nussbaum RL, Murphy EJ. Fatty acid incorporation is decreased in astrocytes cultured from α-synuclein gene-ablated mice. Journal of Neurochemistry. 2005;94(3):839–849. doi: 10.1111/j.1471-4159.2005.03247.x. [DOI] [PubMed] [Google Scholar]
- 12.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 1975;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey RJ, Baskaya MK, Dogan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by Ifenprodil, a polyamine-site N-methyl-d-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47(2):399–406. doi: 10.1097/00006123-200008000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barceló-Coblijn G, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Molecular and Cellular Biology. 2005;25(22):10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis EF, Nies AS, Oates JA. Cerebral arterial smooth muscle contraction by thromboxane A2. Stroke. 1977;8:480–483. doi: 10.1161/01.str.8.4.480. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 17.Fukui S, Fazzina G, Amorini AM, Dunbar JG, Marmarous A. Differential effects of atrial natriuretic peptide on the brain water and sodium after experimental cortical contusion in the rat. Journal of Cerebral Blood Flow and Metabolism. 2003;23:1212–1218. doi: 10.1097/01.WCB.0000088762.02615.30. [DOI] [PubMed] [Google Scholar]
- 18.Ghezzi P, Sacco S, Agnello D, Marullo A, Caselli G, Bertini R. LPS induces IL-6 in the brain and in serum largely through TNF production. Cytokine. 2000;12(8):1205–1210. doi: 10.1006/cyto.2000.0697. [DOI] [PubMed] [Google Scholar]
- 19.Godbout JP, Berg BM, Kelley KW, Johnson RW. α-tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunology. 2004;149:101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Golovko MY, Barceló-Coblijn G, Castagnet PI, Austin S, Combs CK, Murphy EJ. The role of alpha-synuclein in brain lipid metabolism: A downstream impact on brain inflammatory response. Molecular and Cellular Biochemistry. 2009;326(1–2):55–66. doi: 10.1007/s11010-008-0008-y. [DOI] [PubMed] [Google Scholar]
- 21.Golovko MY, Færgeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. α-Synuclein gene-deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of α-synuclein palmitate binding. Biochemistry. 2005;44(23):8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- 22.Golovko MY, Murphy EJ. Brain prostaglandin formation is increased by α-synuclein gene-ablation during global ischemia. Neuroscience Letters. 2008;432:243–247. doi: 10.1016/j.neulet.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golovko MY, Rosenberger TA, Færgeman NJ, Feddersen S, Cole NB, Pribill I, Berger J, Nussbaum RL, Murphy EJ. Acyl-CoA synthetase activity links wild-type but not mutant α-synuclein to brain arachidonate metabolism. Biochemistry. 2006;45:6956–6966. doi: 10.1021/bi0600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golovko MY, Rosenberger TA, Feddersen S, Færgeman NJ, Murphy EJ. α-Synuclein gene ablation increases docosahexaenoic acid incorporation and turnover in brain phospholipids. Journal of Neurochemistry. 2007;101:201–211. doi: 10.1111/j.1471-4159.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- 25.Haring HP, Akamine P, Habermann R, Koziol JA, del Zoppo GJ. Distribution of integrin-like immunoreactivity on primate brain microvasculature. Journal of Neuropathology and Experimental Neurology. 1996;55(2):236–245. doi: 10.1097/00005072-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain, Behavior, and Immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaworowicz DJ, Korytko PJ, Lakhman SS, Boje KMK., Jr Nitric oxide and prostaglandin E2 formation parallels blood-brain barrier disruption in an experimental rat model of bacterial meningitis. Brain Research Bulletin. 1998;46(6):541–546. doi: 10.1016/s0361-9230(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 28.Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P. Tumour necrosis factor-α affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver International. 2010;30:1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 29.Meredith JE, Fazeli B, Schwartz MA., Jr The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Methia N, André P, Hafezi-Moghadam A, Economopoulos M, Thomas KL, Wagner DD. ApoE deficiency compromises the blood brain barrier especially after injury. Molecular Medicine. 2001;7(12):810–815. [PMC free article] [PubMed] [Google Scholar]
- 31.Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observd in the neurovascular unit. Stroke. 2008;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 33.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of α-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinkinase K and formic acid pretreatment. Experimental Neurology. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- 34.Nedrebø T, Reed RK. Different serotypes of endotoxin (lipopolysaccharide) cause different increases in albumin extravasation in rats. Shock. 2002;18(2):138–141. doi: 10.1097/00024382-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Nonaka N, Shioda S, Banks WA. Effect of lipopolysaccharide on the transport of pituitary adenylate cyclase activating polypeptide across the blood-brain barrier. Experimental Neurology. 2005;191:137–144. doi: 10.1016/j.expneurol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos D, Ewans L, Pham-Dinh D, Knott J, Reynolds R. Upregulation of α-synuclein in neurons and glia in inflammatory demyelinating disease. Mol Cell Neurosci. 2006;31:597–612. doi: 10.1016/j.mcn.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. American Journal of Pathology. 1993;143(1):154–163. [PMC free article] [PubMed] [Google Scholar]
- 38.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for α-synuclein in the regulation of dopamine biosynthesis. Journal of Neuroscience. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: Structure of components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 40.Pickard JD, Macdonell LA, Mackenzie ET, Harper AM. Prostaglandin-induced effects in the primate cerebral circulation. European Journal of Pharmacology. 1977;43:343–351. doi: 10.1016/0014-2999(77)90040-1. [DOI] [PubMed] [Google Scholar]
- 41.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan N. Immune-to-brain signaling: How important are the blood-brain barrier-independent pathways? Mol Neurobiol. 2008;37:142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reisenauer CJ, Bhatt DP, Mitteness DJ, Slanczka ER, Gienger HM, Watt JA, Rosenberger TA. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. Journal of Neurochemistry. 2011;117:264–274. doi: 10.1111/j.1471-4159.2011.07198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojanathammanee L, Murphy EJ, Combs CK. Expression of mutant alpha-synuclein modulates microglial phenotype in vitro. J Neuroinflammation. 2011;8(44):1–12. doi: 10.1186/1742-2094-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberger TA, Villacreses NE, Hovda JT, Boestti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. Journal of Neurochemistry. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- 46.Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J Neuroimmune Pharmacol. 2009;4:276–282. doi: 10.1007/s11481-008-9138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ. α-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Procedings of the National Academy of Science, USA. 2001;98(16):9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slivka AP, Murphy EJ, Horrocks LA. Cerebral edema after temporary and permanent middle cerebral artery occlusion in the rat. Stroke. 1995;26:1061–1065. doi: 10.1161/01.str.26.6.1061. [DOI] [PubMed] [Google Scholar]
- 49.Stolp NB, Dziegielewska KM. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathology and Applied Neurobiology. 2009;35:132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 50.Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. Journal of Cerebral Blood Flow and Metabolism. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. Journal of Cerebral Blood Flow and Metabolism. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 52.Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain, Behavior, and Immunity. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by α-synuclein. Neuroscience Letters. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 54.Willard LB, Hauss-Wegrzyniak B, Wenk GL. Pathological and biochemical consequences of acute and chronic neuroinflammation within the basal forebrain cholinergic system of rats. Neuroscience. 1999;88(1):193–200. doi: 10.1016/s0306-4522(98)00216-4. [DOI] [PubMed] [Google Scholar]
- 55.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood-brain barriers permeability during experimental meningitis in the rat. Journal of Clinical Investagations. 1988;82(4):1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Research. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto YL, Feindel W, Wolfe LS, Katoh H, Hodge CP. Experimental vasoconstriction of cerebral arteries by prostaglandins. Journal of Neurosurgery. 1972;37:385–397. doi: 10.3171/jns.1972.37.4.0385. [DOI] [PubMed] [Google Scholar]