Figure 7.
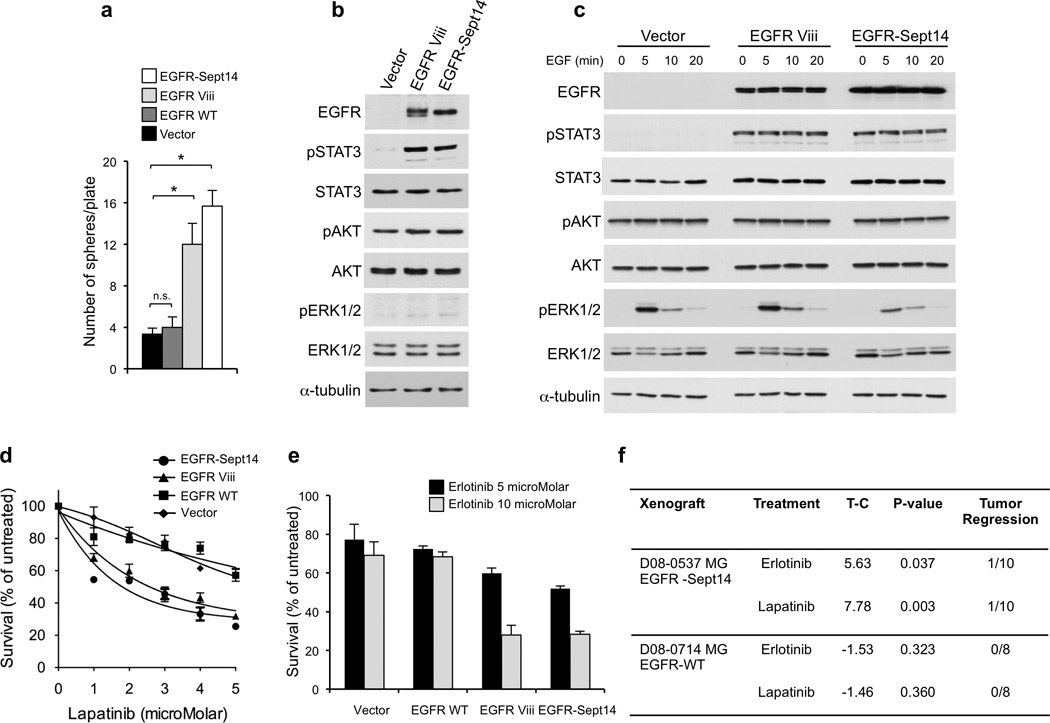
Functional analysis of EGFR-SEPT14 fusion and effect of inhibition of EGFR kinase on glioma growth. a, Sphere forming assay in the absence of EGF of GBM-derived primary cells (#48) expressing vector, EGFR wild type, EGFR Viii or EGFR-SEPT14 fusion. Data are Mean±SD of triplicate samples (t-test, p = 0.0051 and p = 0.027 for EGFR-SEPT14 fusion and EGFR Viii compared with vector, respectively). b, Western blot analysis of GBM-derived primary cells (#48) expressing vector, EGFR Viii or EGFR-SEPT14 fusion cultured in the presence of EGF. c, GBM-derived cells (#48) expressing vector, EGFR Viii or EGFR-SEPT14 fusion were cultured in the absence of EGF for 48 h and then stimulated with EGF 20ng/ml for the indicated time. Cells were assayed by western blot using the indicated antibodies. d, Survival of GBM-derived cells (#48) expressing vector, EGFR wild type, EGFR Viii or EGFR-SEPT14 fusion after treatment with lapatinib for 48 h at the indicated concentrations. Data are Mean±SD of triplicate samples. e, Survival of GBM-derived cells (#48) expressing vector, EGFR wild type, EGFR Viii or EGFR-SEPT14 fusion after treatment with erlotinib for 48 h at the indicated concentrations. Data are Mean±SD of triplicate samples. Experiments were repeated three times. f, In vivo inhibition of tumor growth by EGFR kinase inhibitors in glioma patient derived xenografts carrying EGFR-SEPT14 fusion (n = 10) but not wild type EGFR (n = 8). T-C indicates the median difference in survival between drug treated and vehicle (control) treated mice.
