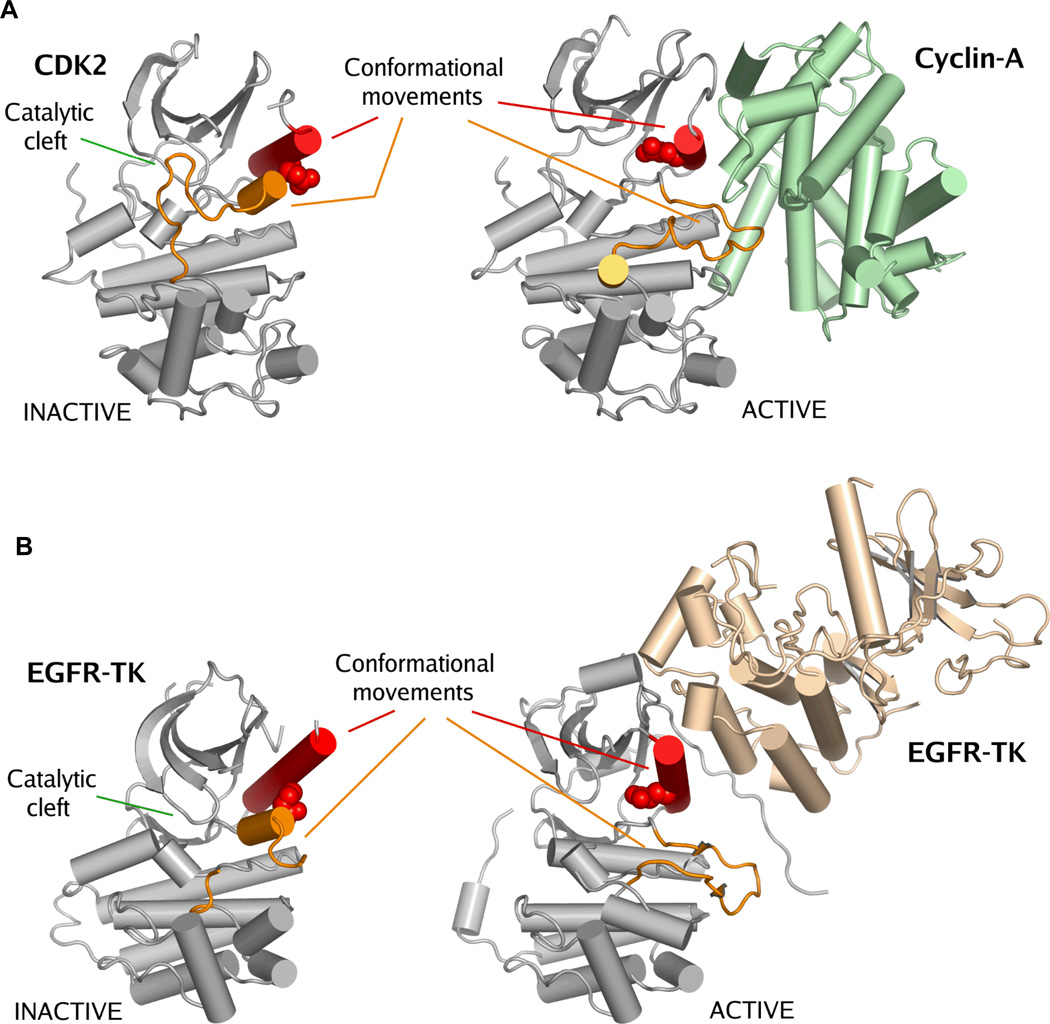
Figure 2. Activation of CDKs and EGFRs occurs by a similar mechanism.
Panel A. Crystal structures of inactive CDK270 (PDB accession code: 1HCK) and cyclin-A in complex with CDK271 (PDB accession code: 1FIN). Panel B. Crystal structures of inactive EGFR tyrosine kinase domain35 (PDB accession code: 1XKK) and active state EGFR-TK36 (PDB accession code: 2GS6). There is a striking similarity between the inactive states of EGFR-TK and CDK2. Activation of these kinases is achieved by a protein-protein interaction that forces a structural rearrangement of the kinase towards the active state. In this figure kinase activation loops are colored orange and the C-helix is colored red. The catalytic glutamic acid is shown as space-filling spheres. The important conformational movements of the C-helix and the activation loop are indicated, but other conformational movements that occur between the active and inactive states are not shown38. The location of the catalytic cleft is indicated. It is important to note that this activating mechanism for wild-type EGFR is different from the disease-associated EGFR mutations.