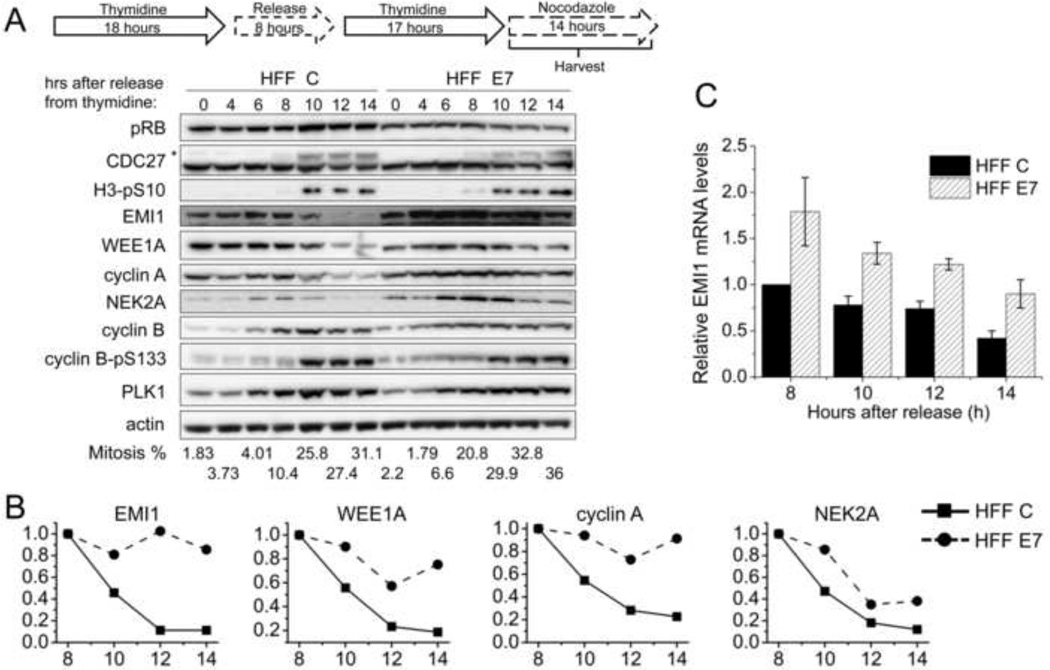
Figure 2. Mitotic EMI1 levels remain high in HPV16 E7-expressing primary human fibroblasts.
(A) HFF C and HFF E7 were synchronized with a double thymidine block and then released into 100 ng/ml nocodazole to trap cells in mitosis. Protein extracts were prepared at the indicated time points after release from the second thymidine block into nocodazole. Western blot analyses of pRB, CDC27, histone H3 phosphorylated at serine 10 (H3-pS10), EMI1, WEE1A, cyclin A, NEK2A, cyclin B, cyclin B phosphorylated at serine 133 (cyclin B-pS133), PLK1 and actin are shown. The asterisk in the CDC27 panel indicates slower migrating, phosphorylated form of CDC27. The results of a representative experiment are shown. Similar results were obtained in 5 experiments. Mitotic indices, as determined by FACS analyses with a parallel set of samples stained with MPM-2 and propidium iodide, are presented below the blots. (B) Quantifications of band intensities of EMI1, WEE1A, cyclin A and NEK2A normalized to actin for the experiment presented in panel A are plotted. Protein levels in HFF C and HFF E7 populations at the 8 hour time points were each set to 1 even though they were not identical in the two cell populations. (C) qRT-PCR analyses of EMI1 mRNA expression in HFF C and HFF E7 that were harvested at 8, 10, 12, 14 hours after release from a double thymidine block into nocodazole. The bar graph shows averages and standard deviations from three independent experiments.