Abstract
Frameless stereotactic neuronavigation provides tracking of surgical instruments on radiographic images and orients the surgeon to tumor margins at surgery. Bipolar electrical stimulation mapping (ESM) delineates safe limits for resection of brain tumors adjacent to eloquent cortex. These standard techniques could complement the capability of intraoperative MR (iMR) imaging to evaluate for occult residual disease during surgery and promote more complete tumor removal. The use of frameless neuronavigation in the high-field iMR imaging suite requires that a few pieces of standard equipment be replaced by nonferromagnetic instruments. Specific use of ESM in a high-field iMR imaging suite has not been reported in the literature. To study whether frameless neuronavigation and electrical stimulation mapping could be successfully integrated in the high-field iMR imaging suite, the authors employed these modalities in 10 consecutive cases involving patients undergoing conscious craniotomy for primary brain tumors located in or adjacent to eloquent cortices. Equipment included a custom high-field MR imaging–compatible head holder and dynamic reference frame attachment, a standard MR imaging–compatible dynamic reference frame, a standard MR imaging machine with a table top that could be translated to a pedestal outside the 5-gauss line for the operative intervention, and standard neuronavigational and cortical stimulation equipment. Both ESM and frameless stereotactic guidance were performed outside the 5-gauss line. The presence of residual neoplasm was evaluated using iMR imaging; resection was continued until eloquent areas were encountered or iMR imaging confirmed complete removal of any residual tumor. Mapping identified essential language (5 patients), sensory (6), and motor (7) areas. The combined use of frameless stereotactic navigation, ESM, and iMR imaging resulted in complete radiographic resection in 7 cases and resection to an eloquent margin in 3 cases. Postoperative MR imaging confirmed final iMR imaging findings. No patient experienced a permanent new neurological deficit. Familiar techniques such as frameless navigation and ESM can be rapidly, inexpensively, safely, and effectively integrated into the high-field iMR imaging suite.
Keywords: brain mapping, intraoperative magnetic resonance imaging, brain tumor surgery, stereotactic techniques
The fundamental goals of brain tumor surgery are to maximize resection of neoplasm and minimize the morbidity that could result from injury to adjacent healthy brain tissue.
Frameless stereotactic neuronavigation has been in use for more than 20 years28 and has proven valuable in guiding the neurosurgeon to the anatomical margin of the tumor.12 Electrical stimulation mapping is a method of delineating functional areas during brain tumor surgery and preventing inadvertent injury to eloquent cortical and subcortical structures.36 Intraoperative MR imaging has been reported to identify residual tumor during surgery, thereby prompting additional tumor resection during the same procedure.1,15 The combined use of neuronavigation, ESM, and high-field iMR imaging has not been previously reported. In this paper we describe our experience using neuronavigation, ESM, and high-field iMR imaging in conscious patients undergoing craniotomy for tumor in, or adjacent to, eloquent cortex. We sought to determine: 1) if we could make minor modifications in our preexisting equipment to permit the use of our standard neuronavigation equipment in the iMR imaging suite; 2) if ESM was technically feasible in the high-field iMR imaging suite; and 3) if iMR imaging would augment these techniques by identifying residual tumor and promoting more complete tumor removal.
Methods
Patient Population
Ten patients (6 men and 4 women, mean age 41 years, range 25–57 years) with symptomatic primary brain tumors in, or adjacent to, eloquent cortex were enrolled in the clinical research protocol, Evaluation and Treatment of Neurosurgical Disorders (NINDS 03-N-0164). Presenting symptoms included seizures (in 7 patients), hemi-paresis (1), headaches (1), loss of balance (1), language disturbances (1), and syncope (1). Tumors were located in the left temporal lobe (in 4 patients), posterior left frontal lobe (1), posterior right frontal lobe (3), left parietal lobe (1), and right parietal lobe (1). The Institutional Review Board of the NINDS approved this research protocol, and informed consent was obtained from each patient.
Imaging Before Surgical Incision
On the day before surgery, fiducial markers (Izi Medical Products, Inc.) were placed on the patients’ scalps and they then underwent MR imaging of the brain with and without contrast using 2D T1-weighted, T2-weighted, FLAIR, and 3D T1-weighted fast-field echo sequences. The 3D imaging series were transmitted by direct cable connection to the surgical navigation system (StealthStation, Medtronic, Inc.). On the day of surgery, local anesthetic was injected into the scalp, a sedative was administered intravenously, and the head was fixed with a 3-pin MR imaging–compatible head clamp (Integra LifeSciences Corp.). The head clamp was linked to the iMR imaging table using a custom-designed MR imaging- compatible table attachment (Integra LifeSciences Corp.) (see Fig. 2A), and a preliminary scan was obtained to confirm imaging quality and adequacy of patient positioning (see Fig. 2D).
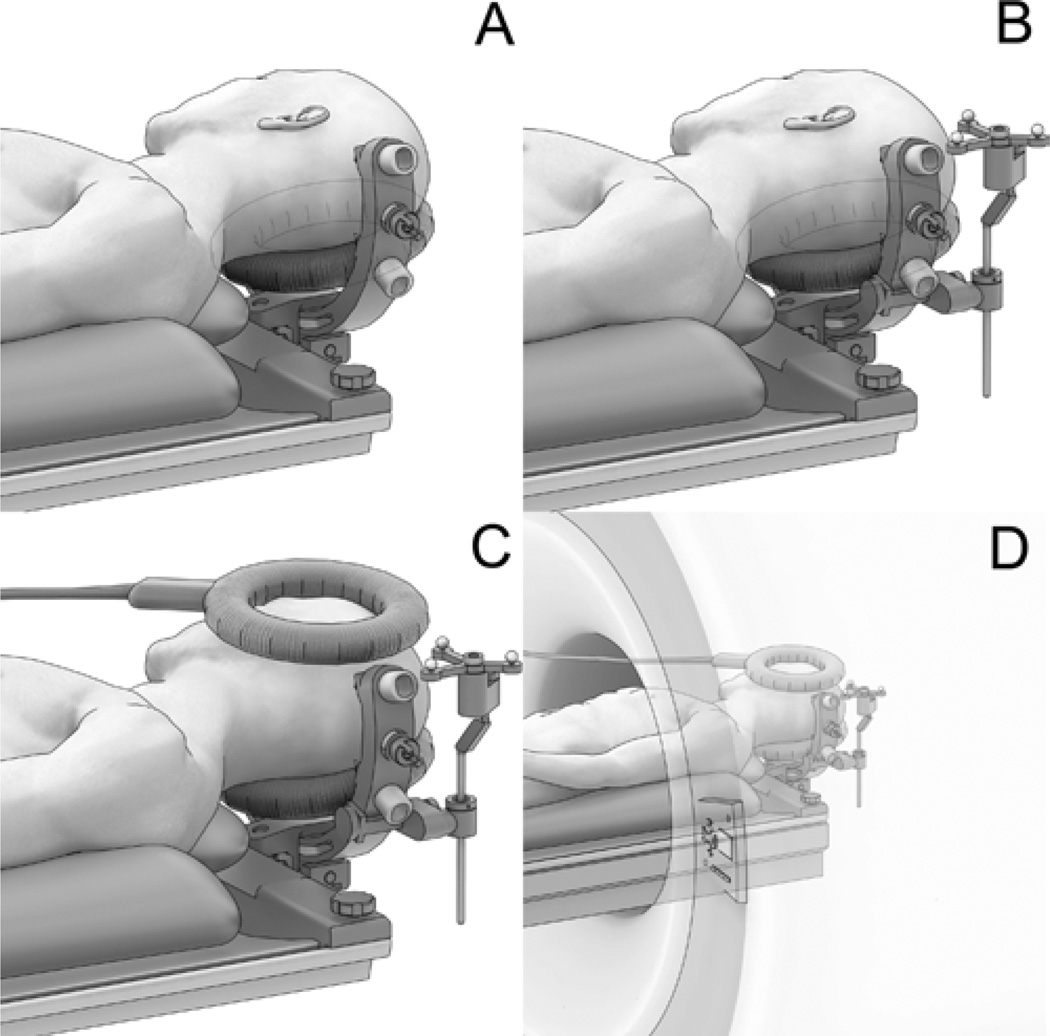
Fig. 2.
Artist’s illustrations. A: Specially designed, MR imaging–compatible, 3-pin head holder extends from end of the operative table and provides convenient access to the surgical site. The MR imaging coil is positioned below the patient’s head throughout the procedure. B: Operative configuration (drapes not shown). An MR imaging–compatible patient reference frame is added for frameless stereotactic navigation. C: Scanning configuration. An additional MR imaging coil is added above the area of interest. D: Configuration within the scanner.
Operative Procedure—Stage 1
All patients underwent craniotomy with local anesthesia and intravenous sedation. The procedure was performed on a modified angiography table within an iMR imaging suite containing a high-field, fixed-position MR imaging unit (Achieva 1.5T, Philips, Andover, MA), similar to the configuration described by Tummala et al.35 The table, fixed outside the 5-gauss line, swiveled into docking position with the MR imaging gantry. The patient was transported from the operating position to the imaging position by docking the angiography table with the iMR imaging gantry and sliding the table top into the MR imaging bore (Fig. 1). An MR imaging–compatible patient reference frame (Polestar, Medtronic, Inc.) was attached to the head holder using an MR imaging–compatible, custom- designed patient reference frame holder (Integra LifeSciences Corp.) (Fig. 2B). The scalp was treated with antimicrobial solution and draping was performed. The neuronavigation system (StealthStation) was registered in a standard manner. The surface representation of underlying tumor margins assisted in selection of an appropriate trajectory and operative corridor.
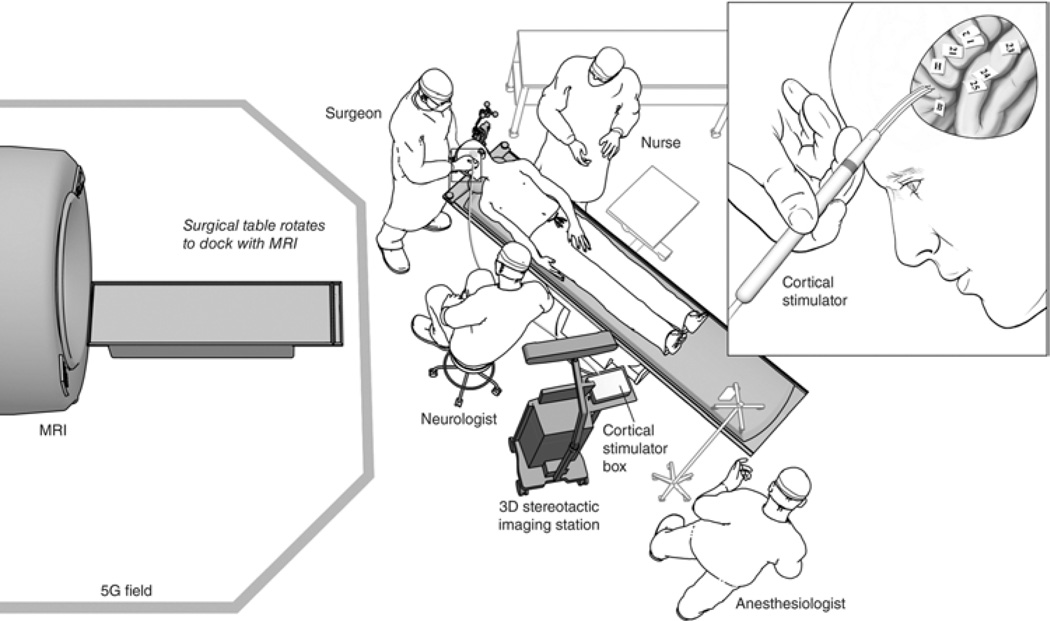
Fig. 1.
Schematic illustration of the iMR imaging suite. Personnel and equipment are organized outside the 5-gauss (5G) field line to permit cortical mapping (inset) and frameless stereotactic navigation. A surgical assistant stands next to the surgeon but is not shown in this illustration.
Intravenous sedation was withdrawn after performing the craniotomy but before opening the dura mater. The dura was opened after the patient became fully conscious and cortical mapping was then performed.
Electrical Stimulation Mapping
All ESM was performed using a bipolar electrical stimulator probe (5 mm between tips) connected to a pulse generator (Ojemann Cortical Stimulator, Integra LifeSciences), which was placed outside the 5-gauss field to prevent electrical interference (Fig. 1). Stimulation was performed over the entire exposed cortical surface using biphasic square wave pulses (0.5 msec per pulse, 50 Hz, 2-second duration) beginning at 2 mA and increasing in 1-mA increments until a functional response was detected. Stimulation intensity did not exceed 10 mA for motor and sensory regions or 16 mA for language. Motor cortex was localized by eliciting movement in the face or limbs. Somatosensory cortex was identified when the patient reported paresthesias in response to cortical stimulation. Essential cortex for receptive language (the Wernicke area) was localized to sites on the cortex where stimulation induced speech arrest and anomia during object naming. Electrical stimulation that resulted in speech arrest during number counting identified cortex for expressive language (the Broca area). Numbered sterile tags were placed over each point of stimulation that elicited a functional response (Fig. 3). Points that did not elicit an effect on stimulation were deemed noneloquent and were covered by tags marked with letters. Digital photographs of the cortical maps were produced; the resulting images were reproduced on a color printer and mounted on a stand near the surgeon. Patients were serially tested for speech, sensory, and motor function throughout the resection. This testing included frequent evaluation of spontaneous movement and strength in the contralateral limbs during removal of tumors that were adjacent to motor cortex.
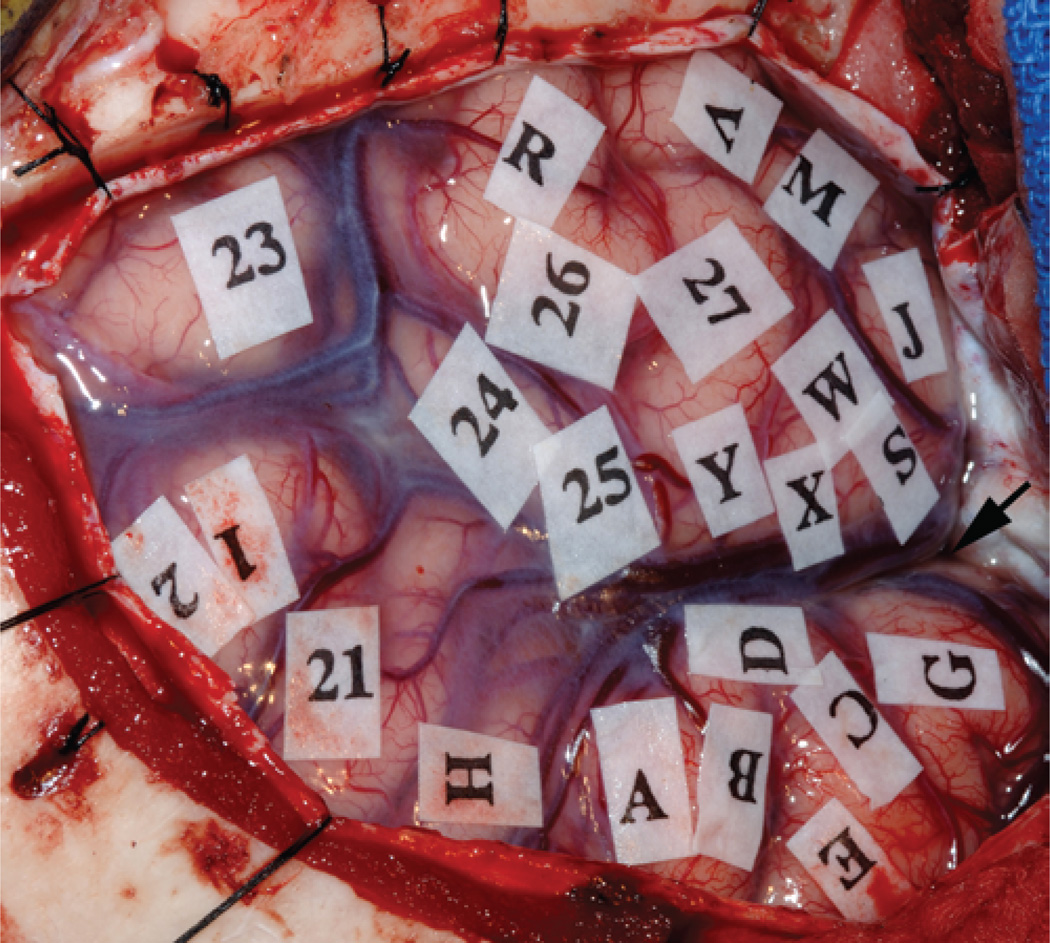
Fig. 3.
Intraoperative photograph showing the view from the surgeon’s perspective after a left frontotemporal craniotomy. The left temporal lobe is in the upper part of the photograph. The sylvian fissure is marked with an arrow. During the mapping process, sterile tags were used to mark eloquent areas of the cortex, which were associated with the following responses to stimulation: 1 and 2, paresthesias in mouth; 21, speech arrest during counting in English (Broca area); 24, errors induced during English confrontation naming test (English Wernicke area); 23–27, speech arrest during Mandarin confrontation naming test (Mandarin Wernicke area). Letters indicate regions that were not associated with any observable response to stimulation.
Operative Procedure—Stage 2
Cortical resection was performed at least 1 cm from areas of motor, sensory, or essential language function as identified by ESM. A neurologist, neuropsychologist, or anesthesiologist monitored motor and speech function throughout the procedure, increasing the frequency of checks when the surgeon approached an identified area of eloquence. Tumor removal was performed by dissecting circumferentially around the border of the tumor as determined by registered preoperative radiographic images displayed on the neuronavigation system and by visual cues of a transitional zone between normal and neoplastic tissue. Resection of the tumor was guided by the surgeon’s intraoperative observation and assessment of tumor margins with assistance from the neuronavigation system (Bronze Probe, Medtronic, Inc.). Prior to iMR imaging to evaluate extent of resection, the dura and skin were loosely approximated. A temporary sterile sheet was placed over the field. Surgical drapes that were hanging below the operative table were trimmed to allow easy passage through the iMR imaging bore.
Imaging During Surgery
For lesions that enhanced on preoperative images, iMR imaging sequences were limited to T1-weighted images with and without contrast medium. For low-grade or nonenhancing lesions, FLAIR and T2-weighted sequences were obtained. Images obtained from the iMR imaging workstation were transmitted by computer network and uploaded to the picture archive retrieval and storage system for evaluation by the neuroradiologist. No other iMR imaging was performed if complete radiographic resection was noted. Conversely, if the neuroradiologist detected residual disease that the surgeon felt could be removed safely, resection continued until the radiographic extent of the tumor was removed or the border of an eloquent area was reached. Confirmation of complete tumor removal in these patients required additional iMR imaging sessions. All patients were evaluated after surgery by MR imaging and neurological examination and were followed up in a longitudinal fashion.
Results
The surgical navigation system accurately localized the tumor and facilitated planning of craniotomy margins in all cases. Intraoperative MR imaging did not cause any demonstrable interference with the neuronavigation system. Similarly, the nonferromagnetic neuronavigation reference frame did not cause any artifact during iMR image acquisition.22 Electrical stimulation mapping identified eloquent cortex in all patients and was not affected by the iMR imaging. Areas of essential language function were identified in 5 patients, sensory areas in 6, and motor areas in 7. Five tumors enhanced and 5 tumors did not enhance with contrast medium administration. After initial resection, iMR imaging identified complete resection in 1 case and residual neoplasm in 9 (Fig. 4). In 2 of the 9 cases of residual tumor no further resection was undertaken because residual tumor invaded areas of eloquence identified by ESM (Wernicke area) or by iMR imaging (posterior limb of internal capsule). The patients in the remaining 7 cases underwent additional tumor resection based on the findings of the iMR imaging. In 6 of these 7 patients tumor resection was continued until it was radiographically complete. In 1 patient additional tumor was removed up to the point where tumor invaded eloquent cortex and white matter, as identified by ESM and iMR imaging.
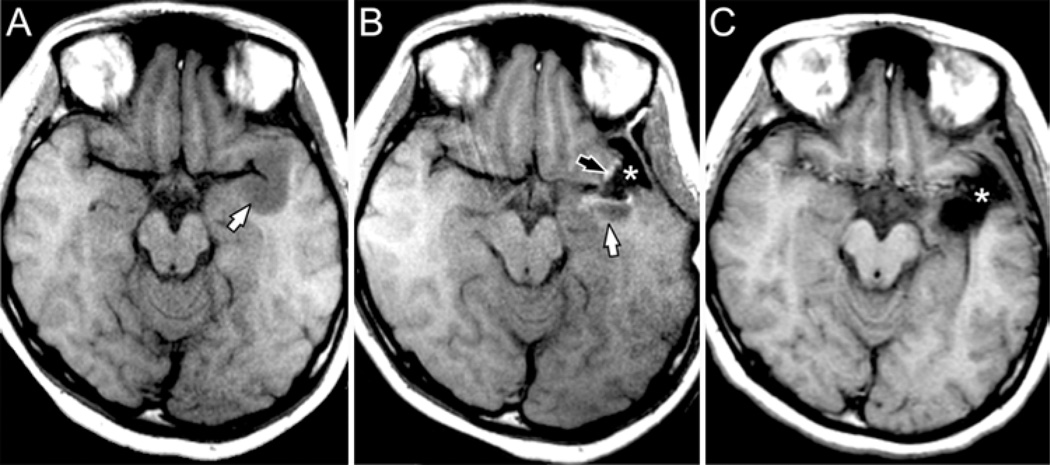
Fig. 4.
Axial T1-weighted images (from the same case as Fig. 3) demonstrating improvement in resection of nonenhancing tumor using iMR imaging guidance. A: Noncontrast image obtained in the iMR imaging scanner immediately prior to craniotomy demonstrates a hypointense mass in the left anterior temporal lobe (white arrow). B: Intraoperative MR image obtained after initial resection documents CSF signal in the resection cavity (asterisk), hyperintense signal representing blood at the margins of the cavity (black arrow), and residual tumor posterior to the resection cavity (white arrow). The operation was extended. C: Postoperative MR image shows extension of the resection cavity (asterisk) to include the region of residual tumor. No residual tumor or blood products can be identified.
Neurological function was unchanged in most patients immediately after surgery. Three patients developed mild speech deficits in the early postoperative period. These resolved to the preoperative baseline after administration of a short course of dexamethasone. The average total operative time was 6.8 hours (range 3.8–8.7 hours). The average number of intraoperative scans was 2 (range 1–3). Each scanning session extended the operating time about 1 hour, as it took 10 minutes to transition between the operating and scanning positions and 30–40 minutes to perform the necessary MR imaging sequences.
Discussion
Magnetic resonance imaging is the standard method used to assess the extent of surgical resection of primary brain tumors. Prior to the advent of iMR imaging technology, extent of tumor resection was gauged during surgery with neuronavigation based on registered preoperative images, real-time ultrasonographic imaging, or surgeon assessment based on personal experience. The first generation of iMR imaging systems used low-field strength magnets (0.12–0.5 T); because resolution is lower on low-field scans than on high-field scans, residual tumor that was imperceptible on intraoperative low-field imaging could be apparent on postoperative, high-field scans (≥ 1.5 T). The introduction of high magnetic field strength iMR imaging suites allowed the neurosurgeon to evaluate for residual tumor during surgery using the same standard used postoperatively. Intraoperative MR imaging provided a method to serially evaluate and improve the extent of resection during the operative procedure, which could avert early reoperation to remove residual tumor.1 Despite these advantages, high-field iMR imaging required movement of either the magnet or the patient for imaging and extended the length of surgery,18 which theoretically increases the risk of infection, deep venous thrombosis, and pulmonary embolism. Interestingly, utilization of iMR imaging has not been reported to increase the rate of these complications.4,15,16,18
The benefit of maximizing resection of primary brain tumors has been a matter of long-standing debate, though evidence is mounting to support aggressive attempts to increase extent of resection. In a recent review of the literature published over the past 18 years, Sanai and Berger29 found significant survival benefit associated with greater extent of resection. Although available data were insufficient for full meta-analysis, their evaluation of available studies showed that, compared with partial resection, complete radiographic resection improved mean survival time from 61 to 90.5 months for patients with WHO Grade II gliomas, from 64.9 to 75.2 months for those with Grade III gliomas, and from 11.3 to 14.2 months for those with Grade IV gliomas. Given the ability of iMR imaging to improve extent of resection, patients treated with the aid of iMR imaging would thus be expected to have a survival advantage compared with patients in whom intraoperative assessment of residual tumor is performed using less sensitive methods. The results of initial studies relating iMR imaging use to survival time have been conflicting, however, and the total number of patients studied in this manner remains small.7,18 Some may question whether a potential but unproven survival benefit supports the cost of building and maintaining an iMR imaging suite.
Avoidance of neurological deficit during brain surgery depends on protecting eloquent cortex and tracts from direct or ischemic injury. Primary motor, somatosensory, and language areas of the cerebral cortex are considered to be eloquent because their removal will result in irreversible and disabling neurological deficits. Penfield and Boldrey23 mapped the motor and sensory homunculi by stimulating the cerebral cortex with a monopolar electrode in conscious patients undergoing brain surgery for medically intractable epilepsy. Bipolar ESM was later used during tumor surgery and for language area localization.36 More recently, ESM has also been used to identify critical subcortical white matter tracts.9,10 In our study, ESM precisely delineated eloquent areas of the cortex in all patients. Other investigators have reported that the risk of transient language deficit is 36% and that of permanent deficit 0% when resection is limited to a distance of more than 1 cm from the nearest language site; with a margin of 0.7–1.0 cm, the risks of transient and permanent language deficit were 91 and 22%, respectively, and with a margin < 0.7 cm, the risks were 100 and 43%.14 Our results support these findings: we maintained a 10-mm margin and avoided permanent neurological morbidity.
Mapping using SSEPs to detect phase reversal across the central sulcus13 can be used in patients who cannot tolerate awake surgery. Likewise, the motor cortex can be mapped using ESM in patients under general anesthesia, but testing under general compared with local anesthesia requires more current and often simultaneous electromyographic monitoring rather than simple visual observation.13 Ideally, techniques could be developed to accurately localize eloquent structures outside the operating room, which would reduce the operative time that is devoted to ESM or SSEP mapping. For identification of primary motor and somatosensory areas, MR imaging can be used to locate the central sulcus as an anatomical landmark.5,37 Functional mapping techniques have been developed, including fMR imaging, PET, diffusion tensor imaging, magnetoencephalography/magnetic source imaging, and SSEPs.2,19,20,27,30 The application of fMR imaging to brain mapping in tumor cases is limited by tumor-related changes in blood oxygen level–dependent (BOLD) signal,11,25 task-dependent variability in language mapping,6 and intraoperative brain shift.3,8,17 In contrast to fMR imaging and other methods that identify the position of functional areas within the cranium based on 3D coordinates derived from landmarks on preoperative images, ESM of the cortex creates a topographic (surface) map of brain function that remains recognizable despite shifting and deformation of the brain. Using ESM as a “gold standard,” reports have shown up to 2 cm inaccuracy in SSEPs and fMR imaging identification of eloquent cortex.24,26,31 Inaccurate mapping of functional areas with methods other than ESM could create inadvertent injury to eloquent structures, whereas overestimation of the extent of essential functional areas with noninvasive techniques could result in suboptimal resection.
In this series of 10 patients undergoing brain tumor surgery in or adjacent to eloquent cortex, neuronavigation, ESM, and iMR imaging were useful individually and in concert.
Neuronavigation and ESM facilitated the identification of pathological and eloquent margins, respectively. In our study, the ability to perform MR imaging in the operating room led to a conservative first resection. At the conclusion of surgery, there was a 70% gross-total resection rate. Total radiographic resection could not be performed in cases in which tumor extended into eloquent cortex. A gross-total resection rate of 35–38% was reported in 2 recent, large series in which iMR imaging was not used.21,32
Conclusions
The tools of stereotactic neuronavigation, ESM, and high-field iMR imaging have been extensively reported in the literature as independent adjuncts to brain tumor surgery. Tools that are developed to enhance the neurosurgeon’s ability to localize normal and pathological tissue should be validated individually and as adjuncts to existing technology.33,34 Stereotactic neuronavigation, ESM, and iMR imaging were found to be complementary tools that promoted maximal resection of neoplastic tissue and preservation of eloquent structures.
Acknowledgment
The authors acknowledge the assistance of Clarissa J. Liew, M.D., who tested and interpreted object naming in Mandarin for one of the patients in this case series.
Disclosure
This research was supported by the Intramural Research Program of the National Institute of Neurologic Disorders and Stroke at the National Institutes of Health.
Abbreviations used in this paper
- ESM
electrical stimulation mapping
- fMR imaging
functional MR imaging
- iMR imaging
intraoperative MR imaging
- NINDS
National Institute of Neurological Disorders and Stroke
- SSEP
somatosensory evoked potential
References
- 1.Albayrak B, Samdani AF, Black PM. Intra-operative magnetic resonance imaging in neurosurgery. Acta Neurochir (Wien) 2004;146:543–557. doi: 10.1007/s00701-004-0229-0. [DOI] [PubMed] [Google Scholar]
- 2.Araujo D, Machado HR, Oliveira RS, Terra-Bustamante V, Barros de Araujo D, Santos AC, et al. Brain surface reformatted imaging (BSRI) in surgical planning for resections around eloquent cortex. Childs Nerv Syst. 2006;22:1122–1126. doi: 10.1007/s00381-006-0063-1. [DOI] [PubMed] [Google Scholar]
- 3.Arbel T, Morandi X, Comeau RM, Collins DL. Automatic non-linear MRI-ultrasound registration for the correction of intra-operative brain deformations. Comput Aided Surg. 2004;9:123–136. doi: 10.3109/10929080500079248. [DOI] [PubMed] [Google Scholar]
- 4.Archer DP, McTaggart Cowan RA, Falkenstein RJ, Sutherland GR. Intraoperative mobile magnetic resonance imaging for craniotomy lengthens the procedure but does not increase morbidity. Can J Anaesth. 2002;49:420–426. doi: 10.1007/BF03017334. [DOI] [PubMed] [Google Scholar]
- 5.Biega TJ, Lonser RR, Butman JA. Differential cortical thickness across the central sulcus: a method for identifying the central sulcus in the presence of mass effect and vasogenic edema. AJNR Am J Neuroradiol. 2006;27:1450–1453. [PMC free article] [PubMed] [Google Scholar]
- 6.Bookheimer S. Pre-surgical language mapping with functional magnetic resonance imaging. Neuropsychol Rev. 2007;17:145–155. doi: 10.1007/s11065-007-9026-x. [DOI] [PubMed] [Google Scholar]
- 7.Claus EB, Horlacher A, Hsu L, Schwartz RB, Dello-Iacono D, Talos F, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 8.Comeau RM, Sadikot AF, Fenster A, Peters TM. Intraoperative ultrasound for guidance and tissue shift correction in image- guided neurosurgery. Med Phys. 2000;27:787–800. doi: 10.1118/1.598942. [DOI] [PubMed] [Google Scholar]
- 9.Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76:845–851. doi: 10.1136/jnnp.2004.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffau H, Lopes M, Sichez JP, Bitar A, Capelle L. A new device for electrical stimulation mapping of the brainstem and spinal cord. Minim Invasive Neurosurg. 2003;46:61–64. doi: 10.1055/s-2003-37961. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara N, Sakatani K, Katayama Y, Murata Y, Hoshino T, Fukaya C, et al. Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors. Neuroimage. 2004;21:1464–1471. doi: 10.1016/j.neuroimage.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Golfinos JG, Fitzpatrick BC, Smith LR, Spetzler RF. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg. 1995;83:197–205. doi: 10.3171/jns.1995.83.2.0197. [DOI] [PubMed] [Google Scholar]
- 13.Gregorie EM, Goldring S. Localization of function in the excision of lesions from the sensorimotor region. J Neurosurg. 1984;61:1047–1054. doi: 10.3171/jns.1984.61.6.1047. [DOI] [PubMed] [Google Scholar]
- 14.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hall WA, Liu H, Martin AJ, Pozza CH, Maxwell RE, Truwit CL. Safety, efficacy, and functionality of high-field strength interventional magnetic resonance imaging for neurosurgery. Neurosurgery. 2000;46:632–642. doi: 10.1097/00006123-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Hall WA, Truwit CL. Intraoperative MR imaging. Magn Reson Imaging Clin N Am. 2005;13:533–543. doi: 10.1016/j.mric.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Hastreiter P, Rezk-Salama C, Soza G, Bauer M, Greiner G, Fahlbusch R, et al. Strategies for brain shift evaluation. Med Image Anal. 2004;8:447–464. doi: 10.1016/j.media.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Hirschberg H, Samset E, Hol PK, Tillung T, Lote K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg. 2005;48:77–84. doi: 10.1055/s-2004-830225. [DOI] [PubMed] [Google Scholar]
- 19.Kakigi R, Hoshiyama M, Shimojo M, Naka D, Yamasaki H, Watanabe S, et al. The somatosensory evoked magnetic fields. Prog Neurobiol. 2000;61:495–523. doi: 10.1016/s0301-0082(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 20.Krings T, Chiappa KH, Foltys H, Reinges MH, Cosgrove GR, Thron A. Introducing navigated transcranial magnetic stimulation as a refined brain mapping methodology. Neurosurg Rev. 2001;24:171–179. doi: 10.1007/s101430100151. [DOI] [PubMed] [Google Scholar]
- 21.McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700–708. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 22.Nimsky C, Fujita A, Ganslandt O, von Keller B, Kohmura E, Fahlbusch R. Frameless stereotactic surgery using intraoperative high-field magnetic resonance imaging. Neurol Med Chir (Tokyo) 2004;44:522–534. doi: 10.2176/nmc.44.522. [DOI] [PubMed] [Google Scholar]
- 23.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 24.Petrovich N, Holodny AI, Tabar V, Correa DD, Hirsch J, Gutin PH, et al. Discordance between functional magnetic resonance imaging during silent speech tasks and intraoperative speech arrest. J Neurosurg. 2005;103:267–274. doi: 10.3171/jns.2005.103.2.0267. [DOI] [PubMed] [Google Scholar]
- 25.Picht T, Wachter D, Mularski S, Kuehn B, Brock M, Kombos T, et al. Functional magnetic resonance imaging and cortical mapping in motor cortex tumor surgery: complementary methods. Zentralbl Neurochir. 2008;69:1–6. doi: 10.1055/s-2007-993138. [DOI] [PubMed] [Google Scholar]
- 26.Pirotte B, Neugroschl C, Metens T, Wikler D, Denolin V, Voordecker P, et al. Comparison of functional MR imaging guidance to electrical cortical mapping for targeting selective motor cortex areas in neuropathic pain: a study based on intraoperative stereotactic navigation. AJNR Am J Neuroradiol. 2005;26:2256–2266. [PMC free article] [PubMed] [Google Scholar]
- 27.Pouratian N, Sheth SA, Martin NA, Toga AW. Shedding light on brain mapping: advances in human optical imaging. Trends Neurosci. 2003;26:277–282. doi: 10.1016/S0166-2236(03)00070-5. [DOI] [PubMed] [Google Scholar]
- 28.Roberts DW, Strohbehn JW, Hatch JF, Murray W, Kettenberger H. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545–549. doi: 10.3171/jns.1986.65.4.0545. [DOI] [PubMed] [Google Scholar]
- 29.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–756. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 30.Seeck M, Michel CM, Spinelli L, Lazeyras F. EEG mapping and functional MRI in presurgical epilepsy evaluation. Rev Neurol (Paris) 2001;157:747–751. [PubMed] [Google Scholar]
- 31.Shinoura N, Yamada R, Suzuki Y, Kodama T, Sekiguchi K, Takahashi M, et al. Functional magnetic resonance imaging is more reliable than somatosensory evoked potential or mapping for the detection of the primary motor cortex in proximity to a tumor. Stereotact Funct Neurosurg. 2007;85:99–105. doi: 10.1159/000098524. [DOI] [PubMed] [Google Scholar]
- 32.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland GR, Latour I, Greer AD. Integrating an imageguided robot with intraoperative MRI: a review of the design and construction of neuroArm. IEEE Eng Med Biol Mag. 2008;27:59–65. doi: 10.1109/EMB.2007.910272. [DOI] [PubMed] [Google Scholar]
- 34.Tan TC, Black PM. Image-guided craniotomy for cerebral metastases: techniques and outcomes. Neurosurgery. 2007;61:349–357. doi: 10.1227/01.neu.0000279228.50826.88. [DOI] [PubMed] [Google Scholar]
- 35.Tummala RP, Chu RM, Liu H, Truwit CL, Hall WA. High-Field functional capabilities for magnetic resonance imaging-guided brain tumor resection. Tech Neurosurg. 2002;7:319–325. [Google Scholar]
- 36.Whitaker HA, Ojemann GA. Graded localisation of naming from electrical stimulation mapping of left cerebral cortex. Nature. 1977;270:50–51. doi: 10.1038/270050a0. [DOI] [PubMed] [Google Scholar]
- 37.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]