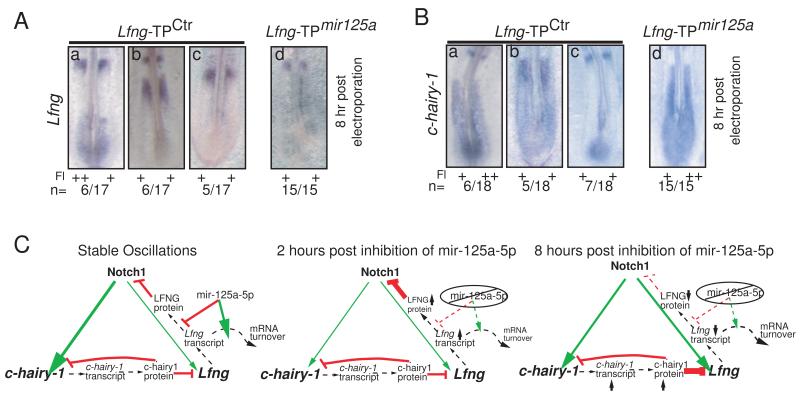
Figure 4.
Long term inhibition of interactions between mir-125a-5p and Lfng perturbs the oscillatory expression of genes linked to the segmentation clock via feedback. A. 8h post-electroporation, cyclic expression of endogenous Lfng is observed in Lfng-TPCtrl embryos (a-c) while endogenous Lfng expression is non-cyclic, with no expression detected in the caudal PSM of Lfng-TPmir-125a positive embryos (panel d, n=15/15). B. 8h post-electroporation, cyclic expression of endogenous chairy1 is observed in Lfng-TPCtrl embryos (panels a-c), but non-cyclic cHairy expression is observed in the caudal PSM of Lfng-TPmir-125a positive embryos (panel d, n=15/15). Fl: Fluorescein; ‘+’ and ‘++’ indicate relative levels of fluorescein signal, images available in Fig S4. C Model for effects of mir-125a-5p in the clock. Stable clock oscillations are governed in part by interlocking feedback loops where Notch activates Lfng and c-hairy-1 (green arrows), while LFNG protein inhibits Notch signalling, and c-hairy-1 protein inhibits its own transcription and that of Lfng (red lines). The lengths of delays imposed by transcription rate and translational efficiency (dashed lines), as well as the half-lives of transcripts and proteins are critical for maintenance of stable oscillations. mir-125a-5p is proposed to increase the rate of Lfng transcript turnover and/or decrease the efficiency of translation. In the absence of mir-125a-5p, (middle) levels of Lfng transcript and LFNG protein increase. In the long term (right), the effect of increased Lfng transcript stability is loss of robust oscillations, with stable, increased levels of c-hairy-1 transcript, and stable decreased transcription of Lfng. See also Fig. S4 for fluorescein images