Summary
The appearance of a mutant androgen receptor, F876L-AR, in prostate cancer cells chronically exposed to enzalutamide or ARN-509 promotes a switch from antagonist to agonist receptor function, undermining the potential long term effectiveness of these second generation anti-androgen drugs.
Prostate cancers become “addicted” to male hormones during the pathogenesis of the disease. In doing so, the cancer cells co-opt androgen receptor (AR) signaling, a driver of secretory cell differentiation in normal prostate cells, for maintenance of a malignant phenotype. Somatic chromosomal translocations and deletions, creating fusions between androgen-regulated differentiation genes and cancer genes, may enable this addiction (1). For more than 70 years, androgen signaling has been targeted for prostate cancer treatment. Initially accomplished via removal of the testes, therapeutic reduction of circulating androgens in men with advanced prostate cancer almost always leads to improvement in disease-related symptoms, to diminution in blood biomarkers of disease activity, and to improvement in radiographic images of disease sites. Unfortunately, for most men, this benefit is short-lived. The disease inevitably progresses despite low levels of circulating androgens to “castration-resistant prostate cancer” (CRPC). CRPC cells often remain addicted to AR signaling, fomenting a recent flurry of new drug discovery and development, already yielding two new approved agents targeting androgen action: the androgen biosynthesis inhibitor abiraterone and the AR antagonist enzalutamide (2).
In the current issue of Cancer Discovery, both Joseph et al. and Korpal et al. report the detection of a mutant AR, resulting from a missense change leading to an amino acid substitution (F876L) in the ligand binding domain (LBD), that conferred resistance to enzalutamide (3, 4). A similar finding has also been described by Balbas et al. (5). AR is a ligand-activated transcription factor normally responsive to testosterone and dihydrotestosterone. AR mutations had been previously described emerging in response to prostate cancer treatment with “first-generation” receptor antagonists, including flutamide and bicalutamide, resulting in changes in the LBD such that the ligand specificity for AR transcriptional activation was broadened, even to include the receptor antagonists themselves (6). Such mutations may have accounted for some cases of “antiandrogen withdrawal” syndrome, where men with progressive prostate cancer despite receptor antagonist treatment appeared to benefit from cessation of therapy (7). However, these mutations did not explain the majority of CRPC cases. Rather, increased AR expression levels, sometimes associated with AR amplification, were found to heighten ligand sensitivity and increase ligand promiscuity to drive CRPC progression (8). This CRPC phenotype motivated the pursuit of “second-generation” AR antagonists, such as enzalutamide and ARN-509, identified using screens for AR inhibition despite high-level AR expression.
To explore mechanisms of resistance to enzalutamide and ARN-509, Joseph et al. selected variants of the LNCaP prostate cancer cell line, and of an LNCaP/AR cell subline engineered to over-express AR, via chronic exposure to the second generation anti-androgens in vitro (3). In 3 of 10 resistant variant sublines, both enzalutamide and ARN-509 exhibited partial AR agonist activity, stimulating both cell proliferation and target gene expression. AR sequencing revealed a missense mutation generating a F876L change in the LBD in each of these sublines. The F876L-AR bound enzalutamide and ARN-509 with 48-fold and 30-fold greater affinity than wild-type AR. Forced expression of this AR mutant in LNCaP cells was sufficient to confer agonist activity to the second generation AR antagonists in vitro and in vivo, likely by permitting a homodimeric association of one N-terminus with helix 12 at the other C-terminus known to form an agonist conformation at AR DNA binding sites.
Joseph et al. then analyzed plasma DNA from a phase 1 clinical trial of ARN-509 for metastatic CRPC (3). Though >40% of men receiving ARN-509 showed declines in serum prostate-specific antigen (PSA) indicating response to treatment, of 29 men available for molecular analysis, 18 ultimately exhibited PSA increases, hinting at intrinsic or acquired resistance to the drug. Using a PCR-based BEAMing method to detect F876L-encoding mutant AR variants, mutant AR sequences (C to A change at nt 2628) were found in plasma DNAs from 3 of the men with progressive cancer despite ARN-509 treatment, while no such variants were present in any of the men before treatment (3). When this association of F876L-AR with prostate cancer progression despite ARN-509 treatment was considered in the context of the agonist activity of ARN-509 in prostate cancer cells expressing F876L-AR, a compelling case for F876L-AR mediating clinical resistance to second generation anti-androgens could be made.
Using a similar approach, Korpal et al. also generated LNCaP variant sublines using prolonged exposure to enzalutamide in vitro, isolating 4 sublines exhibiting resistance to the drug (4). For these sublines, enzalutamide was unable to prevent AR trafficking to the cell nucleus or to abolish expression of AR-regulated genes. Whole transcriptome sequencing disclosed an F876L-AR-encoding mutation in each subline, and transient transfection of cDNA for F876LAR and AR-dependent reporter constructs showed a switch to from antagonist to agonist activity upon exposure to enzalutamide. Predictably, 3 of 4 LNCaP tumor xenografts with acquired resistance to enzalutamide also showed F876L-AR expression. Forced stable expression of F876L-AR conferred enzalutamide-resistant growth to LNCaP, VCaP, and Myc-CaP cells in vitro. Curiously, LNCaP variant sublines carrying F876L-AR grew poorly, if at all, as xenograft tumors in castrated mice in vivo. The growth of such xenograft tumors was nonetheless stimulated by enzalutamide. Examining gene expression data for enzalutamide-resistant sublines, Korpal et al. speculated that persistent expression of ‘cell cycle’ and ‘E2F1 activation’ gene sets might nominate CDK4/cyclin D1 assembly as a candidate therapeutic target for prostate cancers progressing despite second generation anti-androgen treatment. In support of this notion, the enzalutamide-resistant LNCaP sublines appeared sensitive to the CDK4/6 inhibitors LEE011 and PD033299.
The consistent finding of F876L-AR in LNCaP sublines selected for resistance to second generation anti-androgens in both reports, along with the propensity for the F876L-AR to mediate agonist responses to enzalutamide and ARN-509, strongly indicts this mutant receptor as a likely mediator of clinical resistance to this class of drugs. The detection of mutations encoding F876L-AR in men progressing despite treatment with ARN-509 further supports this contention. Ready emergence of treatment resistance has long bedeviled inhibitory or toxic therapy of microorganisms and of human cancers. Luria and Delbrück distinguished between spontaneous and induced mutations as a source of resistance using fluctuation analysis to study phage lysis of bacteria, a formalism recapitulated for cancer by Goldie and Coldman (9, 10). For most acquired anti-neoplastic drug resistance, spontaneous mutation appears to account for generating variant cells capable of growth after initial treatment responses. With spontaneous mutation rates in some cancer cells reported as high as 1 in 104 per cell/generation, drug-resistant variants are nearly certain to be present at the time of advanced cancer treatment (11). Such a scenario is likely to apply to AR variants appearing in response to second generation anti-androgen treatment, where cancer cell burdens are likely on the order of 109 or higher. This may be especially true for prostate cancers with DNA mismatch repair defects, as the LNCaP cells used both by Joseph et al. and Korpal et al. fail to express MSH2 or MSH6 (3, 4, 12). Cytotoxic chemotherapy may also induce copious mutations, which could contribute to anti-cancer drug resistance. However, most men with CRPC have not been exposed to such drugs. Of interest, to activate transcription of target genes, AR recruits TOP2B to its DNA binding sequences, increasing chromosomal translocations triggered by TOP2B double strand breaks (13). While AR-induced translocations could conceivably drive AR amplification, this mechanism seems unlikely to generate missense mutant AR forms like F876L-AR.
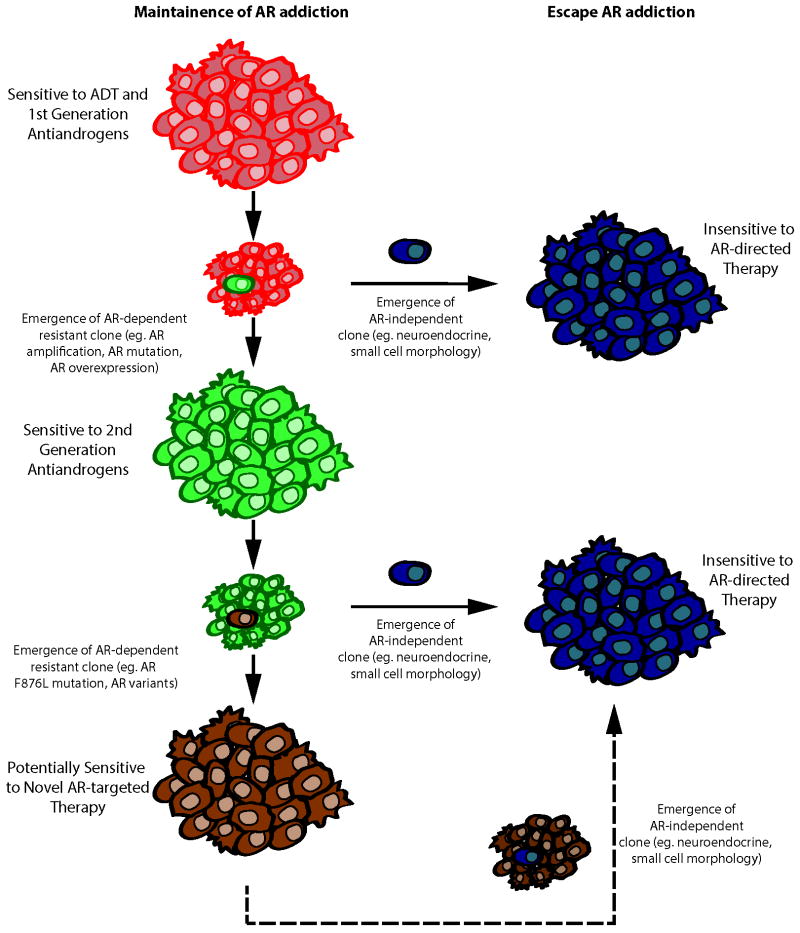
Often new mutations in critical genes subject to selective pressures arise at a significant fitness cost. As an example, while mutant forms of the BCR-ABL fusion gene product can mediate resistance to imatinib in chronic myelogenous leukemia (CML), when imatinib is discontinued, imatinib-sensitive CML cells can reappear (14). Like BCR-ABL for CML, prostate cancer cells exhibit marked addiction to AR. The emergence of CRPC cells with F876L-AR during second generation anti-androgen treatment underscores this addiction. Nonetheless, the poor growth of F876L-AR expressing cells as xenograft tumors in castrate mice seen by Korpal et al. hints at a potential fitness cost of the mutation (4). If this finding anticipates the clinical behavior of CPRC treated with second generation anti-androgens, then discontinuation of treatment might lead to a “second-generation anti-androgen withdrawal” syndrome. Of note, using a similar approach, Balbas et al. did not observe attenuated growth of F876L-AR prostate cancer cells in castrate mice (5). Instead, Balbas et al. employed a combination of molecular modeling, medicinal chemistry, and cell-based screening to define pharmacophores for “next-generation anti-androgens” capable of antagonizing F876L-AR function (5). The promising drug candidates that have been identified by this approach suggest that as long as prostate cancer cells remain addicted to AR signaling, AR can be therapeutically targeted (Figure 1). The more concerning clinical challenge on the horizon will be the tendency for CRPC to end its AR addiction, adopting a more neuroendocrine phenotype unresponsive to AR signaling disruptors, a condition presenting few attractive treatment options (Figure 1)(15).
Figure 1. Mechanisms of Resistance to AR-Directed Therapies.
Despite treatment with androgen deprivation therapy (ADT) or first generation anti-androgens, prostate cancers progress to castration-resistance, often with emergence of an AR-dependent resistant phenotype that is sensitive to treatment with second generation anti-androgens such as enzalutamide or ARN-509. However, AR-dependent resistance emerges again, this time driven by mutant AR. This tendency to maintain AR addiction will permit treatment with next generation AR antagonists. If prostate cancer clones appear at any time during disease progression that have escaped AR addiction, such treatments will prove ineffective.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29(27):3659–68. doi: 10.1200/JCO.2011.35.1916. Epub 2011/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15(15):4792–8. doi: 10.1158/1078-0432.CCR-08-2660. Epub 2009/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to 2nd generation anti-androgens enzalutamide and ARN-509. Cancer Discovery. 2013;X:xx–xx. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 4.Korpala M, Korna JM, Gaob X, Rakiecc DP, Ruddyc DA, Doshia S, et al. A novel mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discovery. 2013;X:xx–xx. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 5.Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife. 2013;2:e00499. doi: 10.7554/eLife.00499. Epub 2013/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20(13):3001–15. doi: 10.1200/JCO.2002.10.018. Epub 2002/06/29. [DOI] [PubMed] [Google Scholar]
- 7.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21(14):2673–8. doi: 10.1200/JCO.2003.11.102. Epub 2003/07/16. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 9.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979;63(11-12):1727–33. Epub 1979/11/01. [PubMed] [Google Scholar]
- 10.Luria SE, Delbruck M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28(6):491–511. doi: 10.1093/genetics/28.6.491. Epub 1943/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tlsty TD, Margolin BH, Lum K. Differences in the rates of gene amplification in nontumorigenic and tumorigenic cell lines as measured by Luria-Delbruck fluctuation analysis. Proc Natl Acad Sci U S A. 1989;86(23):9441–5. doi: 10.1073/pnas.86.23.9441. Epub 1989/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leach FS, Velasco A, Hsieh JT, Sagalowsky AI, McConnell JD. The mismatch repair gene hMSH2 is mutated in the prostate cancer cell line LNCaP. J Urol. 2000;164(5):1830–3. Epub 2000/10/12. [PubMed] [Google Scholar]
- 13.Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42(8):668–75. doi: 10.1038/ng.613. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tipping AJ, Mahon FX, Lagarde V, Goldman JM, Melo JV. Restoration of sensitivity to STI571 in STI571-resistant chronic myeloid leukemia cells. Blood. 2001;98(13):3864–7. doi: 10.1182/blood.v98.13.3864. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 15.Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19(13):3621–30. doi: 10.1158/1078-0432.CCR-12-3791. Epub 2013/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]