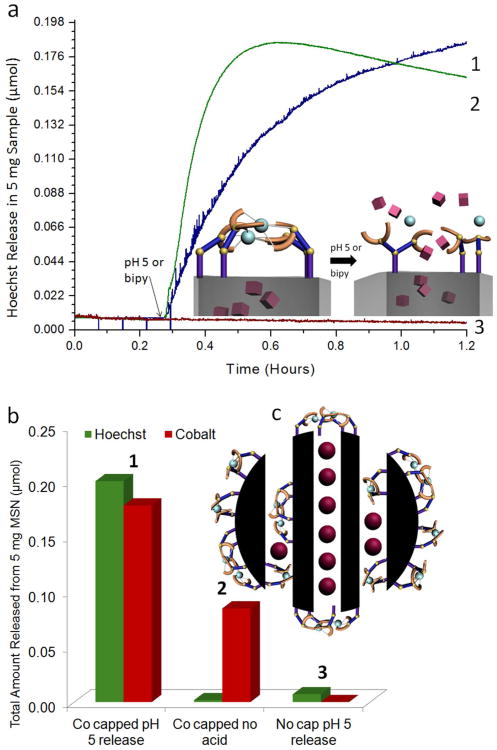
Figure 3.
(a) Time-resolved fluorescence spectra showing the release of Hoechst dye from the gated pores. (Trace 1) Cargo is observed to release from the pores upon changing the solution pH to 5. (Trace 2) Release is also observed when 2,2′-bipyridine is added to the solution, since the competing chelator is capable of removing the metal latch from the nanogate. (Trace 3) No release of Hoechst is observed from Hoechst loaded IDA modified particles when no metal ion is introduced and the nanogate is left unlatched. (b) 1. The total amount of Hoechst and cobalt (in μmols) released from 5 mg of nanomachine. 0.2 μmols of Hoechst released corresponds to a 2% weight release and 1.8 μmols of cobalt released corresponds to a 0.2% weight release. 2. Hoechst loaded, cobalt latched nanoparticles are subjected to a 24 hour soaking period in which a 1% weight release of cobalt is detected, but no Hoechst release. 3. Hoechst loaded IDA modified MSN are “released” by adjusting the solution pH to 5, resulting in a trivial amount of Hoechst released (c) Cobalt ions are released not only from the latched nanogates but also from ligands attached elsewhere on the particle.