Abstract
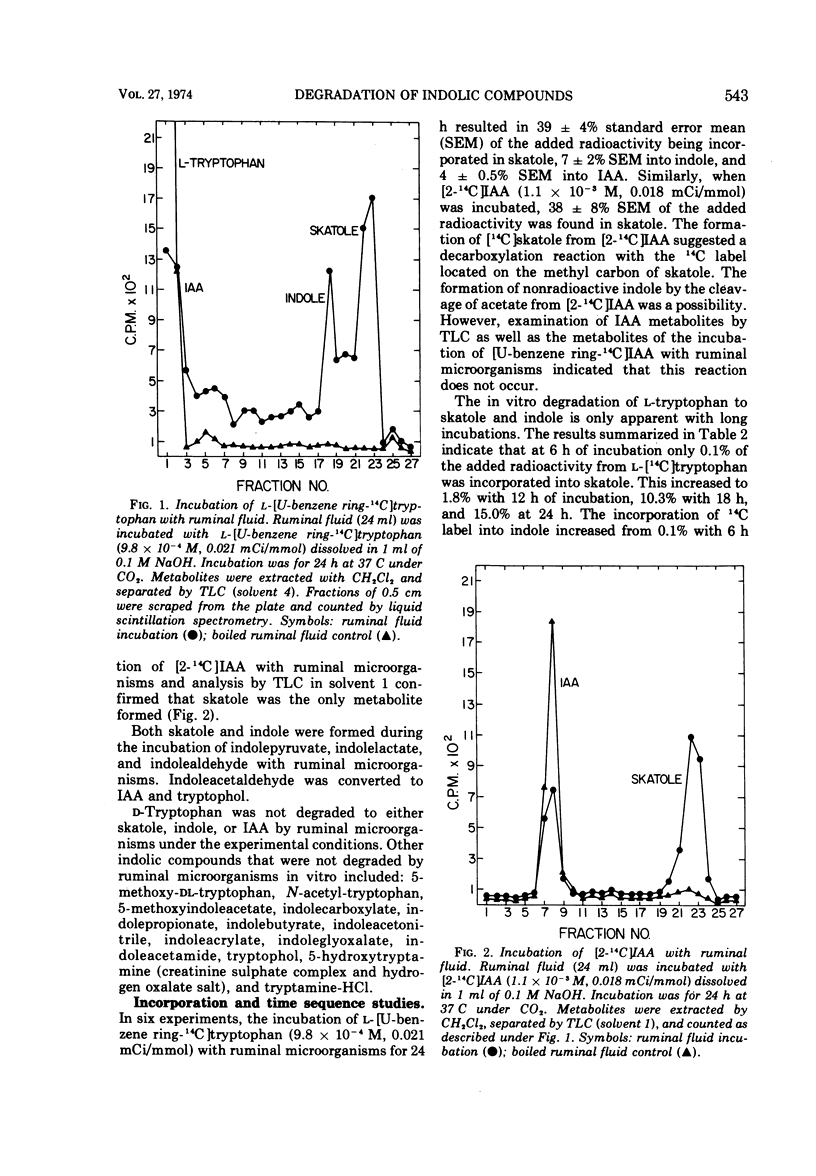
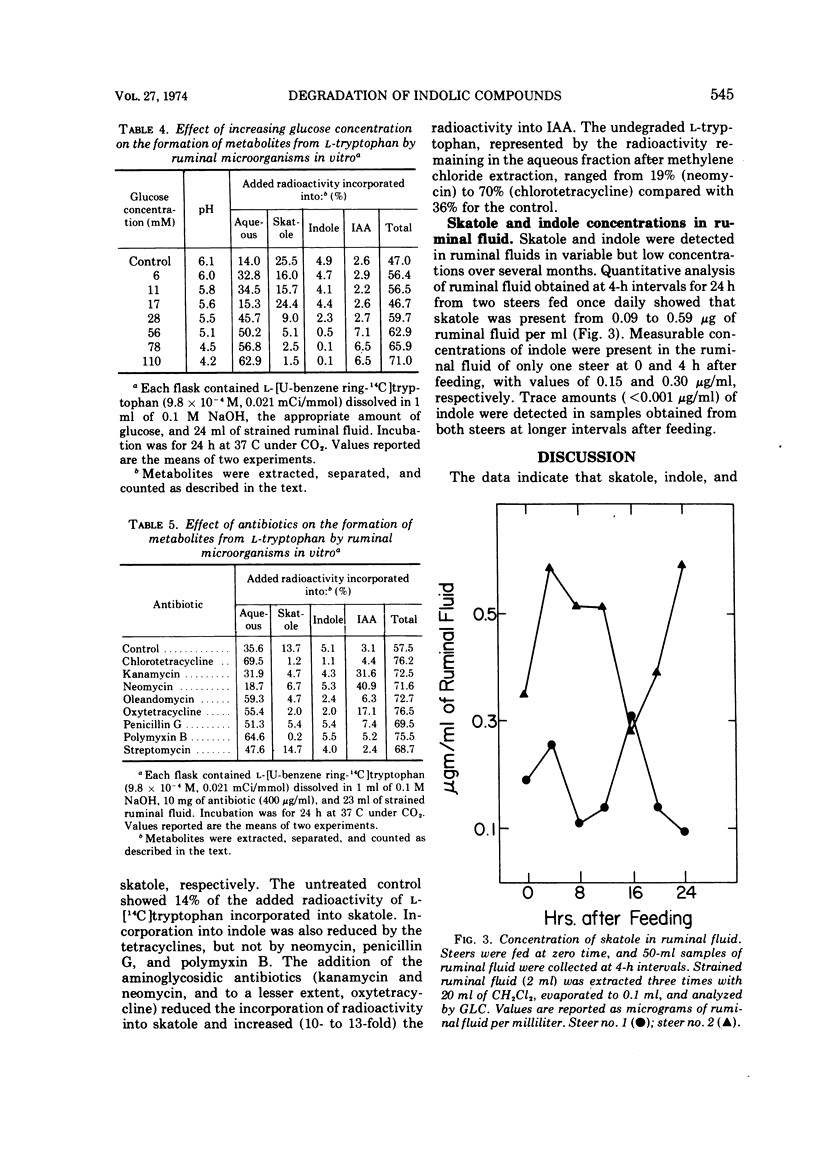
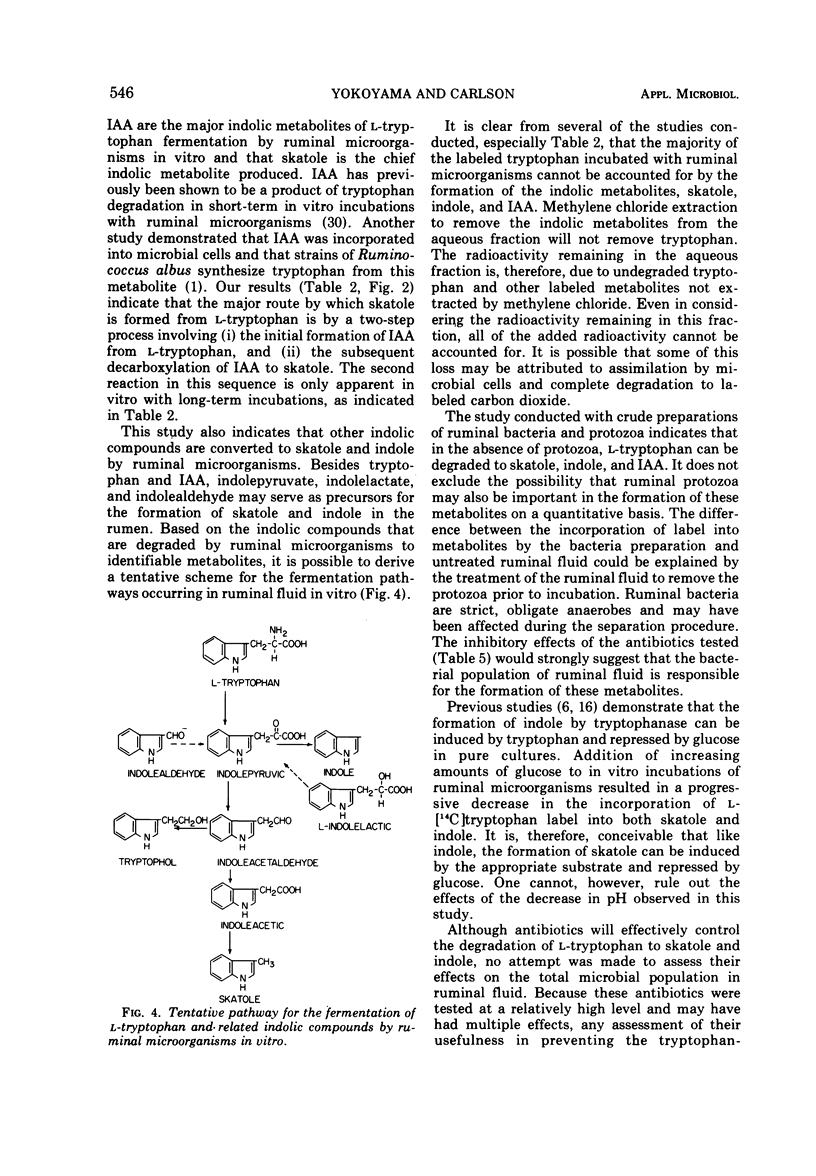
Intraruminal doses of L-tryptophan cause acute pulmonary edema and emphysema in cattle. The D and L isomers of tryptophan and 22 related indolic compounds were incubated with ruminal microorganisms in vitro. Incubation of L-[U-benzene ring-14C]tryptophan with ruminal microorganisms for 24 h resulted in 39% of the added radioactivity being incorporated into skatole, 7% into indole, and 4% into indoleacetate (IAA). D-Tryptophan was not degraded to any of these metabolites. The major pathway of skatole formation from L-tryptophan appeared to be by the decarboxylation of IAA. Incubation of [2-14C]IAA with ruminal microorganisms for 24 h resulted in 38% incorporation into skatole. L-[5-Hydroxy]tryptophan was degraded to 5-hydroxyskatole and 5-hydroxyindole, whereas 5-hydroxyindoleacetate was degraded to only 5-hydroxyskatole. Incubation of indolepyruvate, indolelactate, and indolealdehyde with ruminal microorganisms resulted in the formation of both skatole and indole. Under similar conditions, indoleacetaldehyde was converted to IAA and tryptophol. The addition of increasing concentrations of glucose (0 to 110 mM) reduced the formation of both skatole and indole from L-tryptophan and resulted in the accumulation of IAA. Antibiotics reduced the degradation of L-tryptophan to skatole and indole, with kanamycin and neomycin particularly effective in reducing the decarboxylation of IAA to skatole.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W., HOWARD B. H. Preparation of enzymes from rumen protozoa by indole disintegration. Arch Biochem Biophys. 1962 Nov;99:299–303. doi: 10.1016/0003-9861(62)90014-0. [DOI] [PubMed] [Google Scholar]
- BOYD W. L., LICHSTEIN H. C. The effect of carbohydrates on the tryptophanase activity of bacteria. J Bacteriol. 1955 May;69(5):584–589. doi: 10.1128/jb.69.5.584-589.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. W., Russell G. B. Disruption of protozoa by indole and related compounds. Nature. 1965 Dec 4;208(5014):1001–1002. doi: 10.1038/2081001c0. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Dyer I. A., Johnson R. J. Tryptophan-induced intersitial pulmonary emphysema in cattle. Am J Vet Res. 1968 Oct;29(10):1983–1989. [PubMed] [Google Scholar]
- Carlson J. R., Yokoyama M. T., Dickinson E. O. Induction of pulmonary edema and emphysema in cattle and goats with 3-methylindole. Science. 1972 Apr 21;176(4032):298–299. doi: 10.1126/science.176.4032.298. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., GIBSON D. T. THE BACTERIAL DEGRADATION OF CATECHOL. Biochem J. 1965 May;95:466–474. doi: 10.1042/bj0950466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson E. O., Spencer G. R., Gorham J. R. Experimental induction of an acute respiratory syndrome in cattle resembling bovine pulmonary emphysema. Vet Rec. 1967 Apr 22;80(16):487–489. doi: 10.1136/vr.80.16.487. [DOI] [PubMed] [Google Scholar]
- EADIE J. M., OXFORD A. E. A remarkable disintegrative effect of skatole upon certain rumen ciliate protozoa. Nature. 1954 Nov 20;174(4438):973–973. doi: 10.1038/174973a0. [DOI] [PubMed] [Google Scholar]
- Evans W. C., Handley W. C., Happold F. C. The 'tryptophanase-tryptophan reaction: Possible mechanisms for the inhibition of indole production by glucose in cultures of B. coli. Biochem J. 1942 Apr;36(3-4):311–318. doi: 10.1042/bj0360311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTIERREZ J., WILLIAMS P. P., DAVIS R. E., WARWICK E. J. Lipid metabolism of rumen ciliates and bacteria. I. Uptake of fatty acids by Isotricha prostoma and Entodinium simplex. Appl Microbiol. 1962 Nov;10:548–551. doi: 10.1128/am.10.6.548-551.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEACOCK R. A., MAHON M. E. Paper and thin-layer chromatography of the hydroxyskatoles. Can J Biochem Physiol. 1963 Feb;41:487–496. [PubMed] [Google Scholar]
- HEACOCK R. A., MAHON M. E. THE HYDROXYLATION OF SKATOLE. Can J Biochem Physiol. 1963 Dec;41:2381–2390. [PubMed] [Google Scholar]
- HOGG J. F., ELLIOTT A. M. Comparative amino acid metabolism of Tetrahymena geleii. J Biol Chem. 1951 Sep;192(1):131–139. [PubMed] [Google Scholar]
- Loh H. H., Berg C. P. Production of D-kynurenine and other metabolites from D-tryptophan by the intact rabbit and by rabbit tissue. J Nutr. 1971 Apr;101(4):465–475. doi: 10.1093/jn/101.4.465. [DOI] [PubMed] [Google Scholar]
- Perley J. E., Stowe B. B. The production of tryptamine from tryptophan by Bacillus cereus (KVT). Biochem J. 1966 Jul;100(1):169–174. doi: 10.1042/bj1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K. S. Rabbit erythrocyte hemolysis by lipophilic, aryl molecules. Proc Soc Exp Biol Med. 1969 Apr;130(4):1140–1142. doi: 10.3181/00379727-130-33737. [DOI] [PubMed] [Google Scholar]
- SPISNI D., CAPPA V. Presenza di indolo nel contenuto del rumine di bovini. Boll Soc Ital Biol Sper. 1954 Dec;30(12):1418–1419. [PubMed] [Google Scholar]
- Schatzmann H. J., Gerber H. Production of tryptamine from tryptophan by ruminal fluid in vitro. Zentralbl Veterinarmed A. 1972 Jun;19(6):482–489. doi: 10.1111/j.1439-0442.1972.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Ward P. F., Dawson R. M. The formation and metabolism of phenyl-substituted fatty acids in the ruminant. Biochem J. 1964 Jan;90(1):12–24. doi: 10.1042/bj0900012. [DOI] [PMC free article] [PubMed] [Google Scholar]


