Significance
Adverse social relations in early life are thought to negatively influence health throughout the lifespan. The present findings provide a biological link regarding why negative early life experiences affect health and further suggest that a loving parental figure may provide protection. It is well recognized that providing children in adverse circumstances with a nurturing relationship is beneficial for their overall wellbeing. Our findings suggest that a loving relationship may also prevent the rise in biomarkers indicative of disease risk across numerous physiological systems, impacting adverse health outcomes decades later. The results contribute in a meaningful way to several biological literatures and to the social sciences and, as such, will have a substantial impact.
Keywords: childhood adversity, allostatic load, disease risk
Abstract
Childhood abuse increases adult risk for morbidity and mortality. Less clear is how this “toxic” stress becomes embedded to influence health decades later, and whether protective factors guard against these effects. Early biological embedding is hypothesized to occur through programming of the neural circuitry that influences physiological response patterns to subsequent stress, causing wear and tear across multiple regulatory systems. To examine this hypothesis, we related reports of childhood abuse to a comprehensive 18-biomarker measure of multisystem risk and also examined whether presence of a loving parental figure buffers against the impact of childhood abuse on adult risk. A total of 756 subjects (45.8% white, 42.7% male) participated in this ancillary substudy of the Coronary Artery Risk Development in Young Adults Study. Childhood stress was determined by using the Risky Families Questionnaire, a well-validated retrospective self-report scale. Linear regression models adjusting for age, sex, race, parental education, and oral contraceptive use found a significant positive relationship between reports of childhood abuse and multisystem health risks [B (SE) = 0.68 (0.16); P < 0.001]. Inversely, higher amounts of reported parental warmth and affection during childhood was associated with lower multisystem health risks [B (SE) = −0.40 (0.14); P < 0.005]. A significant interaction of abuse and warmth (P < 0.05) was found, such that individuals reporting low levels of love and affection and high levels of abuse in childhood had the highest multisystem risk in adulthood.
Early-life “toxic” stress increases adult risk for poorer mental health and greater disease morbidity and mortality (1–7). According to a report from the Centers for Disease Control and Prevention (7), several types of childhood toxic stress contribute to this risk, including experiencing traumatic events, physical, sexual or emotional abuse, and growing up in low socioeconomic conditions. Likewise, recent evidence suggests that risky family dynamics characterized as neglectful or harsh parenting and chaotic home life can also influence adult health outcomes (8, 9). Importantly, the most toxic childhood stressors are those that occur in the absence of emotional support from a caregiver (7).
McEwen and Seeman posit that, when the stress response is elicited frequently and/or when it remains activated for a prolonged period, there are excessive demands on these regulatory systems to maintain allostasis, the process of maintaining stability during changing conditions (10). Such demands lead to a state of chronic dysregulation across multiple systems, termed allostatic load. Toxic childhood stress may alter stress response patterns through more frequent elicitation, excessive activation, and alterations in the counterregulatory capacity to down-regulate activity, all of which may contribute to chronic alterations in these regulatory systems (10–12). In support of this, evidence of such forms of allostatic load have been observed across a number of physiological regulatory systems in relation to childhood toxic stress, including increased levels of hemoglobin A1c, elevated total cholesterol, higher adiposity, and metabolic syndrome (13–15). Likewise, neuroendocrine changes have been observed in individuals reporting childhood stress, such as greater sympathetic nervous system (SNS) activity, disrupted hypothalamic–pituitary–adrenal (HPA) activity, and autonomic imbalance (11, 16). Childhood toxic stress has also been associated with a less healthy cardiovascular system profile (e.g., elevated blood pressure), increases in inflammation suggestive of proinflammatory immune system programming, and accelerated cellular aging (17–25). In addition to alterations in adult levels of these biomarkers of risk, several of these markers have been observed in children with stress (18, 23, 26–30), with one study reporting more pronounced effects of cumulative childhood stress on stress hormones, blood pressure, and fat deposition among those with mothers who were cold and unresponsive to their needs (27). This work indicates that the impact of childhood stress on these regulatory systems may begin in childhood, but might be buffered by a nurturing relationship with an adult.
Despite the considerable evidence that childhood toxic stress is associated with worse regulation across multiple biological systems in adulthood, research to date has largely focused on each of these separate, individual systems. Few studies have sought to evaluate the cumulative biological “toll” of childhood stress on adult health through a multisystem (rather than individual-system) perspective, which is captured by the concept of allostatic load (10). Current literature includes links between low childhood socioeconomic status (SES), a risk factor for exposure to toxic childhood environments, and higher scores on a multisystem allostatic risk index in adults (31) and emerging adults (32). However, to date, there has been no research examining qualitative features of the childhood environment that may contribute to the development of allostatic load seen in adulthood. Our analyses examine associations of the quality of the parental relationship with multisystem risk in adulthood, independent of childhood SES. We investigate negative (i.e., abuse) and positive (i.e., warmth and affection) features of the child–parent relationship in childhood, and their interaction, to examine whether parental warmth can buffer against childhood abuse experiences in impacting adult allostatic load.
The unique features of the present analyses include the focus on relationships with parents in childhood as it relates to adult biological risk for disease by using a comprehensive 18-biomarker measure of multisystem risk, i.e., allostatic load. Furthermore, parental warmth is thought to buffer against childhood stress impacting psychological and physical health outcomes (15, 33, 34), but no research to date has tested this hypothesis in an epidemiological sample of adults by using a multisystem index of physiological functioning. The present study examines whether parental warmth buffers against the effects of childhood abuse on allostatic load in adulthood.
Results
Descriptive statistics for all variables are provided in Table 1. Allostatic load scores ranged from 0 to 16.8 (skewness = 0.69, kurtosis = 0.06). A regression analysis with age, sex, race, parental education, and oral contraceptive use (OCU) entered simultaneously in the model revealed that black race [B (SE) = −1.2 (0.25), P < 0.001] and lower parental education [B (SE) = −0.11 (0.05), P < 0.05] predict higher allostatic load scores. Age, sex, and OCU were not significantly associated with allostatic load.
Table 1.
Descriptive statistics for demographic, health behaviors, childhood factors, and allostatic load (N = 756)
| Characteristic | Value |
| Age, y | 40 ± 3.6 |
| White race, % | 45.8 |
| Male sex, % | 42.7 |
| Parental education, y | 13.2 ± 2.7 |
| Average annual family income, $ | 65,078 ± 29,987 |
| Participant education, y | 14.9 ± 2.4 |
| Physical activity, exercise units | 356.3 ± 298.7 |
| Self-reported sleep duration, h per night | 6.5 ± 1.3 |
| Drink alcohol, % | 50.5 |
| Alcohol consumption in drinkers, mL/d | 19.3 ± 25.1 |
| Smoking status, % | |
| Current smoker | 17.7 |
| Past smoker | 17.2 |
| Never smoked | 65.1 |
| Risky family scale | 11.8 ± 4.1 |
| Risky family subscales | |
| Childhood abuse | 1.5 ± 0.7 |
| Parental warmth | 3.2 ± 0.8 |
| Allostatic load (18-item) | 4.8 ± 3.2 |
Values presented as mean ± SD or percentages where applicable.
Childhood Stress and Allostatic Load.
Regression analyses were performed to examine the age-, sex-, race-, parental education-, and OCU-adjusted associations of childhood stress and allostatic load. Risky family environment [B (SE) = 0.10 (0.03), P < 0.001], childhood abuse [B (SE) = 0.68 (0.16), P < 0.001], and parental warmth [B (SE) = −0.40 (0.14), P < 0.005] were all significant predictors of allostatic load in the initial model (model 1). Adjustment for adult SES (model 2), which was a significant independent predictor of allostatic load [B (SE) = −0.82 (0.15), P < 0.001], did not alter the primary associations of risky family environment, childhood abuse, or parental warmth with allostatic load (all P < 0.05). There were no significant interactions of race or sex with childhood stress.
Interaction of Childhood Abuse with Parental Warmth.
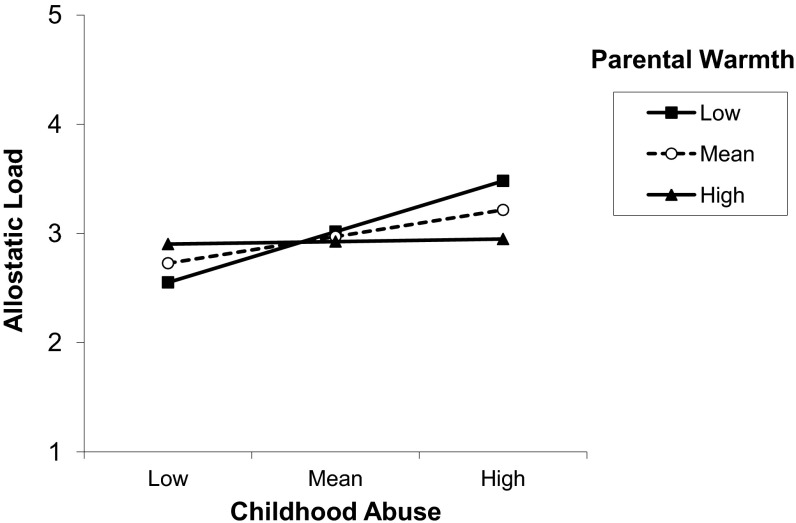
To test the hypothesis that childhood abuse would be more strongly associated with adult allostatic load in those who reported less parental warmth compared with those who reported more parental warmth, we tested for an interaction. In model 1, entering childhood abuse and parental warmth (centered) on the second step, followed by the interaction term childhood abuse by parental warmth on the third step, we found a significant interaction [B (SE) = −0.42 (0.18), P < 0.05]. Further adjustment by adult SES did not alter the findings (P < 0.05). To display the interaction of childhood abuse by parental warmth in the prediction of allostatic load, we graphed the simple slope for childhood abuse predicting allostatic load at 1 SD above the mean of parental warmth, the mean for parental warmth, and 1 SD below the mean for parental warmth (Fig. 1) (35). At 1 SD below the mean of parental warmth (i.e., low warmth), the slope is steepest, suggesting that the strongest relationship between childhood abuse and allostatic load is in those individuals with low parental warmth scores.
Fig. 1.
Simple slopes of childhood abuse predicting allostatic load for 1 SD below the mean of parental warmth (low), the mean of parental warmth (mean), and 1 SD above the mean of parental warmth (high), adjusted for age, sex, race, parental education, OCU, and adult SES.
In SI Results, we report on analyses examining the strength of these associations after further adjustment by lifestyle risk factors. In addition, we report on the unique association of childhood stress with individual biological system risk scores (Table S1).
Discussion
Higher amounts of childhood abuse and lower levels of parental warmth are associated with elevated allostatic load in young and middle adulthood. The strength of the association of childhood abuse withstood adjustments for age, sex, race, OCU, parental education, and adult SES. Furthermore, there was an interaction between parental warmth and childhood abuse, such that those individuals reporting having a parent who showed love and affection were somewhat protected from the effects of childhood abuse on lower allostatic load. In this way, parental warmth may act as a buffer against the effects of toxic childhood stress on allostatic load. These findings suggest that individuals who report experiencing emotional or physical abuse as a child with little or no parental love and affection represent a subgroup at particularly elevated risk for disease via chronic dysregulation across multiple systems (i.e., allostatic load). Our findings are consistent with the hypothesis that one of the mechanisms through which childhood toxic stress contributes to adult risk for disease and death is by repeated disruptions to normal physiological patterns, eventually leading to chronic alterations in set points across multiple biological regulatory systems (9, 36, 37). In addition, these findings support the hypothesis that parental warmth can buffer against childhood toxic stress and reduce the risk for adult disease by reducing the extent of biological wear and tear, captured by our measure of allostatic load.
The present findings raise a question as to how childhood stress would influence adult allostatic load decades later. Current theory postulates that toxic stress may become embedded to influence health decades later, proposing that, during important developmental stages in childhood neural circuitry and peripheral regulatory systems are programmed to respond in a manner that would fit optimally with ecological demands, termed a predictive adaptive response (PAR) (36, 38). The PAR works to provide survival benefits and superior fitness in an environment where dangers are great (e.g., predators, competition for resources) (36). Childhood toxic stress programs the PAR by altering neural systems related to stress responding (34, 39–41), enhancing anticipation of threat, generating greater emotional and physiological arousal to actual threat, and reducing the ability of the system to shut off the stress response. This programming may also have long-term health costs (1, 8, 36, 42–46). We posited that such demands lead to a state of chronic dysregulation across multiple systems. In this way, childhood toxic stress, which alters the stress response patterns with more frequent elicitation, excessive activation, and inadequate counterregulatory responses to turn it off, would contribute to allostatic load (10–12). Our findings suggest that this may indeed be a pathway to disease, with effects occurring across regulatory systems, and independent of childhood and adult SES.
Limitations, Strengths, and Future Research Directions.
One concern in regard to limitations of the present study is that a retrospective childhood stress measure captures recall bias. However, we and others have previously established reliability and validity of retrospective reports of parental relationships and abuse (47–49), validating with interview based measures of childhood (50). Our assessment of childhood abuse and parental warmth does not capture all types of abuse or the multiple facets of parental warmth and affection. Future work should include more comprehensive assessment of abuse and parental warmth.
A limitation to our findings is that our analyses are cross-sectional, reducing our ability to infer causation. Third factors may also exist that were not captured in the present analyses that may explain the relationship observed; these include factors with direct effects on regulatory systems, such as nutrition in the home, environmental pollution, or shared genetics that increase the propensity toward verbal and physical aggression and cause alterations in systems physiology. Likewise, because the analyses are cross-sectional, we cannot rule out the possibility that shifts in regulatory systems influences recall of childhood events, with the potential of higher allostatic load biasing toward recall of more negative childhood events. However, there is no evidence or theory to date that would support an argument that allostatic load engenders recall biases.
One outstanding question is whether sources of warmth come from the same parent that is abusive or from a second parent. Although there is a negative correlation between abuse and warmth (r = −0.45, P < 0.001) in the present sample, there were cases of both high abuse and high warmth. Further research should disentangle the sources of abuse and warmth within and outside the home. This type of information could inform targeted interventions for abused children similar to a recent family-based therapeutic intervention with children in foster care showing improvements in developmental, physical, and mental health outcomes (51). Similarly, resilience factors may also be important, and future work should consider components of the psychosocial environment (e.g., social support, optimism, trait disposition) that may act as buffers.
A remaining question is whether such targeted interventions could be viewed as disease prevention strategies, with such efforts potentially improving long-term health trajectories of these children as they reach later life. If the present findings are substantiated by further research evidence, customized disease prevention strategies might be implemented to target individuals with a history of childhood abuse to address possible multisystem imbalances before disease onset.
Conclusions
In summary, children who experienced emotional and physical abuse and limited love and affection in their early family environment have greater biological risk across multiple biological systems in adulthood. These effects are independent of childhood and adult SES. Importantly, the influence of childhood abuse on adult allostatic load was moderated by parental warmth, such that those with more parental warmth showed no association of childhood abuse with adult allostatic load, whereas those with limited parental warmth had the strongest positive association of childhood abuse with allostatic load. This supports the hypothesis that parental warmth acts as a protective factor, buffering against the harmful effects of toxic childhood stress on health. Notably, these increased multisystem biological imbalances among those with childhood toxic stress is likely involved in the pathophysiology of several age-related diseases, pointing to plausible mechanisms through which early adversity is embedded biologically to increase vulnerability for disease and death decades later.
Materials and Methods
Participants.
The present analyses include a subsample of 844 participants from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a biethnic, longitudinal study of cardiovascular risk beginning in young adulthood. This parent study initially recruited 5,115 black and white male and female subjects from four cities across the United States: Birmingham, AL; Oakland, CA; Minneapolis, MN; and Chicago, IL. There was a balanced recruitment of black and white subjects, male and female subjects, higher and lower education categories (high school education vs. no high school education), and age categories (18–24 y, 25–30 y). Subjects were followed at years 2, 5, 7, 10, and 15. Each site obtained institutional review committee approval and informed consent was obtained for each examination. The present sample is derived from those recruited at the Oakland, CA and Chicago, IL sites, with initial invitations to participate in an ancillary study of biological and physiological markers sent to 721 subjects from Oakland, CA and 615 subjects from Chicago, IL. From this, 844 (63%) agreed to be participants in this substudy, which included a second clinical visit at year 15 to assess SNS activity, HPA axis, and heart rate variability (HRV). The present analyses include 756 subjects (age range, 32–47 y) who had at least 14 of the 18 biomarkers. Compared with the larger CARDIA cohort, this subsample did not significantly vary by age, sex, race, or educational attainment.
Procedures.
For the 15-y CARDIA visit, participants were asked to arrive for the examination having fasted for 12 h, refrained from exercise the day of the examination, and to have not smoked within the last 30 min of examination initiation. All participants completed informed consent, had their blood drawn, provided 12-h overnight urine samples, had their anthropomorphic measurements taken, and had seated blood pressure readings taken. Health behaviors and health history interviews were given, including assessment of medication use (OCU in women; blood pressure and lipid lowering medications, diabetes medication) (24), and participants were asked to complete a series of self-report questionnaires including the psychosocial scales used in the present analyses. (Further details of CARDIA methodology are available at www.cardia.dopm.uab.edu.) The ancillary visit was performed during a second visit, with details on this methodology published previously (52). Briefly, after informed consent, participants were asked to perform a 30-min computer-based HRV recording, instructed on collection of a 12-h overnight urine sample, and salivary cortisol samples over the following day.
Measures.
Risky family environment.
Risky family environment was measured by using a seven-item version of the Risky Families Questionnaire, adapted from Felitti et al. (53), and designed to capture the respondent’s family environment before they were 18 y of age. Response options vary from 1 (indicating “rarely or none of the time”) to 4 (indicating “most or all of the time”) indicating to what extent they felt loved, were shown affection, were verbally or physically abused, lived with a substance abuser, lived in a household that was organized and managed well, and had adults who “knew what they were up to”. Reliability and validity of this measure have been confirmed (50). Items with positive meaning were reverse scored for the seven-item scale, and all items were summed so higher scores reflect greater risky family environment (score range, 7–28). We derived two subscales from the Risky Families Questionnaire. First, to estimate childhood abuse, we averaged scores from two items in the Risky Family Environment scale: (i) how often adult pushed, grabbed, shoved, or hit you; and (ii) how often adult swore at you, insulted you, or put you down. Second, to assess parental warmth, we averaged scores from two items representative of amount of love and affection received by a parent figure: (i) how often they received physical affection and (ii) how often they felt loved, with higher scores reflecting more love and affection.
Childhood SES.
Childhood SES was estimated by using participant report of educational attainment in years of schooling of the father and/or mother (averaged if both available).
Multisystem cumulative biological risk.
We derived a cumulative biological risk index, termed allostatic load, which was designed to capture parameters of major biological regulatory systems (52). This included 18 different biological markers of risk: resting systolic and diastolic blood pressure, heart rate, low-frequency HRV, high-frequency HRV, urinary norepinephrine and epinephrine, AM rise in cortisol (i.e., difference between waking and 45 min postwaking cortisol levels) and cortisol slope, HDL, LDL, triglycerides, glucose, insulin, waist circumference, C-reactive protein (CRP), fibrinogen, and IL-6. Detailed information regarding each biomarker is provided in SI Materials and Methods. Each biomarker was assigned a risk score of 1 or 0 according to whether the value fell within the highest-risk quartile of the biomarker distribution (Table S2 provides descriptive information on biomarkers and high-risk cutpoints). Whether and how medication use should be incorporated into assessments of allostatic load remains an open question. Based on available data, we scored those reporting use of medications for diabetes, or to lower blood pressure or lipids, as high risk (i.e., given a 1 for each if they did not already fall within the high risk quartile for glucose, insulin, systolic or diastolic blood pressure, or LDL, respectively). This scoring is based on the premise that use of medications is an indicator that the individual has a history of poorer biological regulation, which can be considered a component of cumulative system wear and tear. Consistent with previous research (31, 52, 54), quartile risk designations were used to address the unavailability of established clinical risk guidelines for many of the biomarker indicators in the allostatic load score. Nonetheless, quartile risk cutpoints are often similar to conventional clinical risk cutpoints for a number of biomarkers for which such data are available (Table S2) (31, 54). Scores were calculated for participants with data on at least 14 of the 18 biomarkers (i.e., missing four or fewer). (Of those included in the analyses of allostatic load, there were 126 subjects missing data for one biomarker, 79 with missing data for two, 69 with missing data for three, and 17 with missing data for four.) The summary score was computed by taking the mean of the available 0/1 risk indicators and multiplying by 18 to obtain an equivalent scale range for those participants who might be missing data on one to four biomarkers. The possible range of scores is from 0 to 18. [We also examined a version of allostatic load using clinical high-risk cutpoints where available (waist circumference ≥102 cm in men and ≥88 cm in women, triglycerides ≥200 mg/dL, HDL ≤40 mg/dL in men and ≤50 mg/dL in women, LDL ≥160 mg/dL, CRP ≥3 mg/L, systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and glucose ≥126 mg/dL) rather than quartile cutpoints. Parallel results were found using this alternative scoring approach; results are reported in the paper based on the original, quartile-based approach.]
Individual system risk scores.
For each system represented by biological parameters, we calculated a risk score by using the following groupings based on a previous confirmatory factor analysis (11): metabolic risk (waist circumference, triglycerides, HDL, LDL, glucose, insulin), inflammation risk (CRP, IL-6, fibrinogen), blood pressure risk (systolic and diastolic blood pressure), HRV risk (low- and high-frequency HRV, heart rate), SNS hormone risk (epinephrine and norepinephrine), and cortisol risk (AM rise and diurnal slope).
Adult socioeconomic status.
Adult SES was estimated by using standardized scores for years of education and average household income assessed at year 15. Participants reported average household income from a set of 11 income categories (e.g., <$5,000, $5,000–$11,999, $12,000–$15,999 … $50,000–$74,999, $75,000–$99,999, $100,000+), which were converted into continuous variables by replacing the category number with the median for that bracket (e.g., $50,000–$74,999 = $62,500), then standardized. Standardized scores were then summed to create a composite adult SES index.
Lifestyle.
Lifestyle risk factors include measures of sleep quantity, physical activity, smoking, and alcohol use. Detailed information regarding each lifestyle risk factor measurement is provided in SI Materials and Methods.
Data Analysis.
All analyses were conducted by using SPSS software (version 21; IBM). Descriptive statistics were calculated by using the entire cohort. Next, by using linear regression, we conducted tests of linear associations in levels of the overall index of allostatic load with the risky family scale, childhood abuse, and parental warmth. B-Values reflect unstandardized coefficients and SE reflect standard error. Initial model 1 analyses include age, sex, race, parental education, and OCU. We included a measure of childhood SES (parental education) in the initial model to test for effects of childhood family environment, abuse, and love that were independent of childhood SES. To further adjust for adult factors that may contribute to allostatic load scores, we adjusted for adult SES in model 2. In model 3, we included covariates in models 1 and 2 along with lifestyle risk factors (smoking, physical activity, alcohol use, sleep duration) that may act as pathways to disease (SI Materials and Methods). Interaction analyses were performed by first entering the two predictors (centered), followed by the interaction term. Secondary analyses using linear regression were conducted testing for linear trends in the six “system-level” indices reflecting HRV, blood pressure, inflammation, metabolism, SNS, and HPA activity across our childhood stress measures and entering age, sex, race, parental education, and OCU as covariates in these models.
Supplementary Material
Acknowledgments
The CARDIA study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama (Birmingham, AL) (Grants N01-HC95095 and N01-HC48047); University of Minnesota (Grant N01-HC48048); Northwestern University (Grant N01-HC48049); Kaiser Foundation Research Institute (Grant N01-HC48050); University of California (Irvine, CA) Echocardiography Reading Center (Grant N01-HC-45134); Harbor–University of California (Los Angeles, CA) Research Education Institute and Computed Tomography Reading Center (Grant N01-HC-05187). This study was also supported by MacArthur Research Network on Socioeconomic Status and Health through grants from the John D. and Catherine T. MacArthur Foundation; and by Grant T32-MH19925 and the Cousins Center for Psychoneuroimmunology at the University of California (Los Angeles, CA) (J.E.C.). This manuscript has been reviewed by CARDIA for scientific content and consistency of data interpretation with previous CARDIA publications.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315458110/-/DCSupplemental.
References
- 1.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson G, Devaney J, Spratt T. The impact of adversity in childhood on outcomes in adulthood: Research lessons and limitations. J Soc Work. 2010;10:369–390. [Google Scholar]
- 3.Danese A, et al. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pesonen A-K, Räikkönen K. The lifespan consequences of early life stress. Physiol Behav. 2012;106(5):722–727. doi: 10.1016/j.physbeh.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32(7):824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health Committee on Early Childhood, Adoption, and Dependent Care Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 7.Middlebrooks JS, Audage NC. The Effects of Childhood Stress on Health Across the Lifespan. Atlanta: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 8.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128(2):330–366. [PubMed] [Google Scholar]
- 9.Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Dev Psychopathol. 2011;23(3):921–938. doi: 10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 11.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 12.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- 13.Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: A systematic review. BMC Public Health. 2010;10:525. doi: 10.1186/1471-2458-10-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosom Med. 2005;67(6):846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- 15.Miller GE, et al. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22(12):1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21(1):31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 17.Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011;25(7):1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, et al. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol. 2009;28(3):338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drury SS, et al. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Mol Psychiatry. 2011;17(7):719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janicki-Deverts D, Cohen S, Matthews KA, Jacobs DR., Jr Sex differences in the association of childhood socioeconomic status with adult blood pressure change: The CARDIA study. Psychosom Med. 2012;74(7):728–735. doi: 10.1097/PSY.0b013e31825e32e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Needham BL, Fernandez JR, Lin J, Epel ES, Blackburn EH. Socioeconomic status and cell aging in children. Soc Sci Med. 2012;74(12):1948–1951. doi: 10.1016/j.socscimed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 27.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev Psychol. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 28.Brody GH, et al. Supportive family environments, genes that confer sensitivity, and allostatic load among rural African American emerging adults: A prospective analysis. J Fam Psychol. 2013;27(1):22–29. doi: 10.1037/a0027829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke CH, et al. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73(7):533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews KA, Gump BB, Block DR, Allen MT. Does background stress heighten or dampen children’s cardiovascular responses to acute stress? Psychosom Med. 1997;59(5):488–496. doi: 10.1097/00006842-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Gruenewald TL, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74(1):75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans GW, Kim P. Childhood poverty and young adults’ allostatic load: the mediating role of childhood cumulative risk exposure. Psychol Sci. 2012;23(9):979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- 33.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2010;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver ICG, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- 36.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Ann N Y Acad Sci. 1999;8624:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 38.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 39.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 40.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 42.Barker DJP. Mothers, Babies, and Health in Later Life. 2nd Ed. Edinburgh, UK: Churchill Livingston; 1998. [Google Scholar]
- 43.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 44.Epel ES. Telomeres in a life-span perspective: A new “psychobiomarker”? Curr Dir Psychol Sci. 2009;18:6–10. [Google Scholar]
- 45.Shanks N, Lightman SL. The maternal-neonatal neuro-immune interface: Are there long-term implications for inflammatory or stress-related disease? J Clin Invest. 2001;108(11):1567–1573. doi: 10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor SE, Repetti RL, Seeman TE. What is an unhealthy environment and how does it gets under the skin? Annu Rev Psychol. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- 47.Parker G. The Parental Bonding Instrument: Psychometric properties reviewed. Psychiatr Dev. 1989;7(4):317–335. [PubMed] [Google Scholar]
- 48.Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: A reappraisal of retrospective reports. Psychol Bull. 1993;113(1):82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- 49.Dill DL, Chu JA, Grob MC, Eisen SV. The reliability of abuse history reports: A comparison of two inquiry formats. Compr Psychiatry. 1991;32(2):166–169. doi: 10.1016/0010-440x(91)90009-2. [DOI] [PubMed] [Google Scholar]
- 50.Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Pers. 2004;72(6):1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 51.Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeman T, et al. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. Am J Hum Biol. 2010;22(4):463–472. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 54.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.