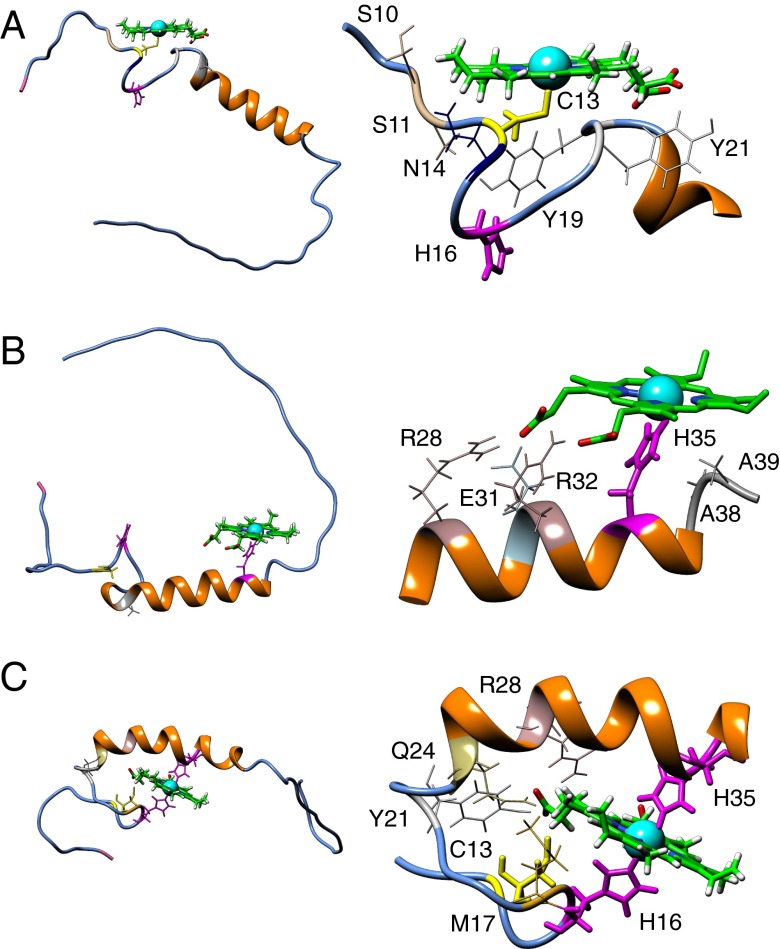
Fig. 6.
Docking of heme to an NMR-derived model of Pep61. (Left) Full-length Pep61 is depicted with secondary structure elements without the cloning overhangs; heme, the residues C13 (yellow), as well as H16 and H35 (magenta) are presented using sticks. The N terminus of the peptide (M1) is shown in pink; residue A23 used for the introduction of a tryptophan for fluorescence quenching experiments shown in Fig. 5 A–C is shown in gray. (Right) Magnified section of the peptide focusing on the heme-binding site. Residues that are presumably taking part in heme ligation are shown in thick lines. (A) Mutant H35A with penta-coordination via C13. (B) WT Pep61 with penta-coordination via H35. (C) WT with hexa-coordination via H16 and H35.