Fig. 4.
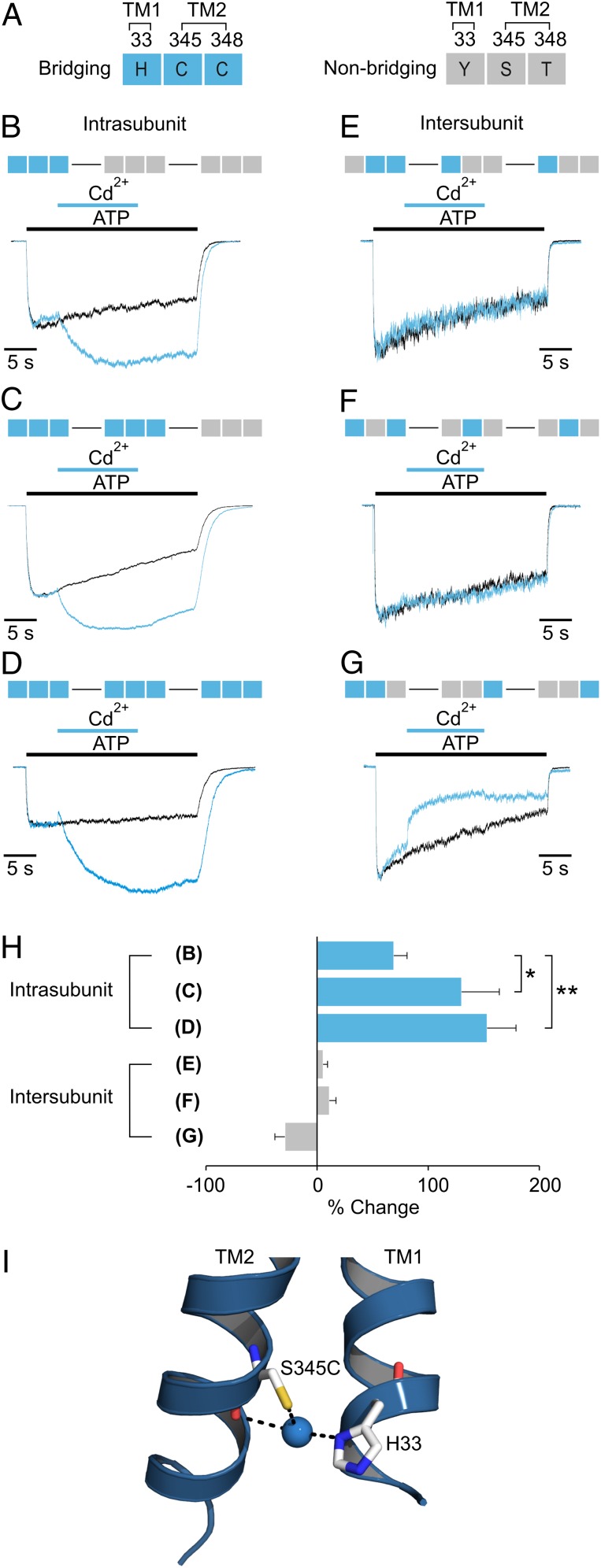
Subunit relationships of metal bridges. (A) Legend for concatenated subunit constructs. Each rectangle (made up of three squares) corresponds to one subunit of the trimer, and the residues at the three bridging positions (33, 345, and 348) are represented by their one-letter amino acid code in each square of the rectangle. Bridging residues (H33, S345C, and C348) are colored blue; nonbridging residues (H33Y, S345, and C348T) are colored gray. (B–D) Concatenated H-C-C subunit constructs with one, two, or three subunits containing all three residues necessary for bridging. Control currents activated by ATP alone (black trace) or ATP plus Cd2+ (blue trace) are superimposed for comparison. (E–G) Concatenated H-C-C subunit constructs with the three residues necessary for bridging split between subunits to test for intersubunit bridges. In each construct, one of the three bridging residues was removed from subunit 1 and placed in the two adjacent subunits. Control currents activated by ATP alone (black trace) or ATP plus Cd2+ (blue trace) are superimposed for comparison. (H) Bar graph summarizing the effects of Cd2+ on each concatameric construct (n = 3–4). Measurements of statistical significance are based on unpaired Student t tests; *P ≤ 0.05, **P ≤ 0.005. See Table S1 for concentration–response relations for each construct. EC 50 concentrations of ATP and 20 µM Cd2+ were used for each construct. (I) Modeling of a Cd2+ bridge between S345C and H33 in the 3T construct of rP2X2 receptor (7) using the X-ray structure of the apo zfP2X4 receptor. In 3T rP2X2 a native Cys at 348 and two additional Cys residues in the C and N termini were mutated to Thr. Bridging residues S345C and H33 (rP2X2 numbering) are shown for only one subunit, with Cd2+ represented as a blue sphere. The side view shown here is from the side opposite that depicted in Fig. 2.