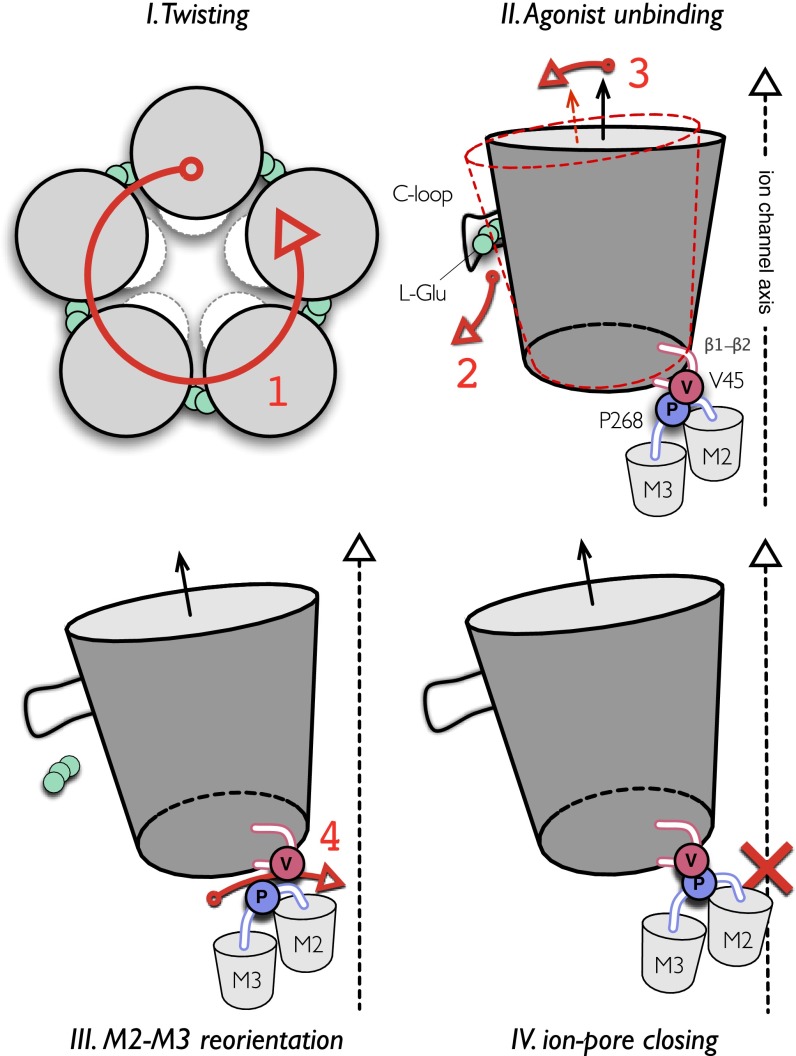
Fig. 6.
Model of the allosteric mechanism for gating ions in pLGICs. The simulation of GluCl with ivermectin removed shows that the closing of the ion pore involves four sequential events, here highlighted by large red numbers. The process is initiated by a global quaternary transition to a twisted conformation of the receptor (red 1). The spontaneous unbinding of l-glutamate from the orthosteric site is the next step (red 2). Agonist unbinding, which is mediated by the opening of the C-loop, results in a significant reorientation of the β-sandwiches in the EC domain that tilt in the outward direction (red 3). This rearrangement leads to the repositioning of the β1–β2 loop at the EC/TM domain interface that opens the way for the passage of the bulky side chain of P268 (on the M2–M3 loop) past V45 at the tip of the β1–β2 loop. The latter leads to an inward displacement of the M2–M3 loop, which results in an outward-to-inward untilting of the pore-lining helices to shut the ion pore (red 4). The closed ion channel produced by the repositioning of M2 is indicated by a red cross in IV. The proposed mechanism assigns a primary role to receptor twisting, which mediates the structural communication between the orthosteric site and the ion pore in pLGICs (see Results).