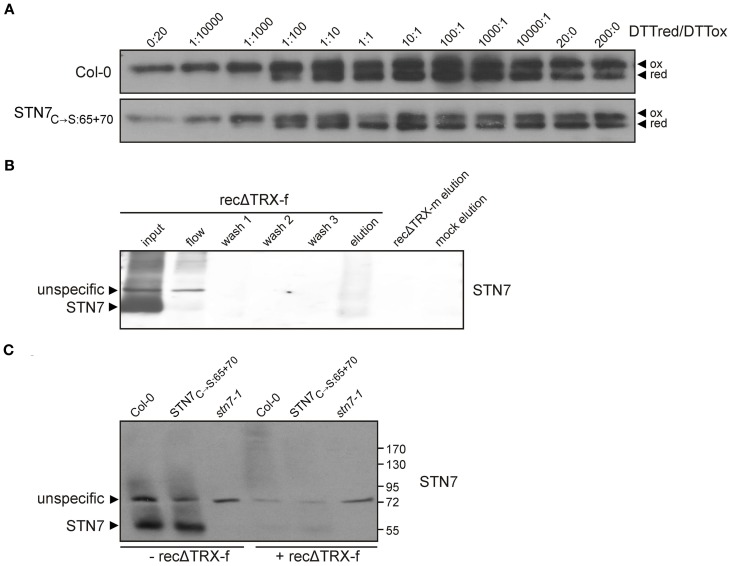
Figure 4.
Redox sensitivity of STN7. (A) Redox titration of STN7 variants. Thylakoids of WT (Col-0) and STN7C→S:65 + 70 were equilibrated at various redox potentials using the DTTred/DTTox redox couple. After separation by non-reducing SDS-PAGE, STN7 was detected by immunoblotting. DTTred/DTTox ratios are indicated above each lane. Oxidized (ox) and reduced (red) forms of the STN7 monomer are indicated by black arrowheads. (B) STN7—TRX-f interaction assayed by thioredoxin affinity chromatography. His-tagged recombinant thioredoxins f and m, with one cysteine replaced by serine (recΔ TRX-f, -m), were bound to Ni-NTA and incubated with thylakoids solubilized with 1.5% digitonin corresponding to 2 mg of Chl. After several washes, the eluates from recΔ TRX-f and -m resins were subjected to Western blot analysis using STN7-specific antibodies. Solubilized WT (Col-0) thylakoids (input; corresponding to 10 μg of Chl), the flow through off the recΔ TRX-f resin (flow; equivalent to 10 μg Chl), washes 1–3 of the recΔ TRX-f resin and the eluate from a similarly treated Ni-NTA resin not coupled to recΔ TRX (mock elution) were loaded as controls. (C) TRX-f mobility shift assay. Thylakoid membranes (10 μg Chl) from WT (Col-0), STN7C→S:65 + 70 and stn7-1 mutants were solubilized with 0.2% DOC and incubated with either 25 μg of recΔ TRX-f or without additives. Proteins were separated by non-reducing SDS-PAGE and Western analysis was performed using STN7-specific antibodies.