Abstract
Background
IABPs are frequently used to provide hemodynamic support during high risk percutaneous coronary intervention (PCI), but clinical evidence to support their use is mixed. We examined hospital variation in IABP use among high risk PCI patients, and determined the association of IABP use on mortality in this population.
Methods and Results
We analyzed data submitted to the CathPCI Registry® between January 2005 and December 2007. High risk PCI was defined as having at least one of the following features: unprotected left main artery as the target vessel, cardiogenic shock, severely depressed left ventricular function, or ST segment elevation myocardial infarction. Hospitals were categorized into quartiles by their proportional use of IABP. We examined differences in in-hospital mortality across hospital quartiles using a hierarchical logistic regression model to adjust for differences in patient and hospital characteristics across hospital quartiles of IABP use. IABPs were used in 18,990 (10.5%) of 181,599 high risk PCIs. Proportional use of IABP varied significantly across hospital quartiles: Q1: 0.0%–6.5%; Q2: 6.6% to 9.2%; Q3: 9.3% to 14.1%; and Q4: 14.2% to 40.0%. In multivariable analysis, after adjustment for differences in patient and hospital characteristics, in-hospital mortality was comparable across quartiles of hospital IABP usage (Q1: Ref; Q2: Odds Ratio (OR) 1.11, 95% CI 0.99–1.24; Q3: OR 1.03, 95% CI 0.92–1.15; Q4: OR 1.06, 95% CI 0.94–1.18).
Conclusion
IABP use varied significantly across hospitals for high risk PCI. However, this variation in IABP use was not associated with differences in in-hospital mortality.
Keywords: Angioplasty, Atherosclerosis, Heart assist device
Introduction
Among patients undergoing percutaneous coronary intervention (PCI), intra-aortic balloon pumps (IABP) are used in a variety of high risk clinical scenarios ranging from patients with cardiogenic shock to those undergoing PCI of high risk lesions (e.g., unprotected left main coronary artery). However, studies of IABP use in PCI patients have yielded conflicting results. Although a number of studies suggested benefits to IABP use in these populations 1–6, other studies- including randomized trials of routine IABP use in patients undergoing primary PCI-have demonstrated no benefit 7–11, and even harm 12, 13. Citing the lack of high quality evidence, the 2005 American College of Cardiology/American Heart Association PCI guidelines do not make specific recommendations for IABP use, but suggest it be considered for hemodynamic support in select high risk patients based on the clinical judgment of the operator14.
At present, there are few data on national patterns of IABP use in high risk PCI in contemporary clinical practice. Furthermore, there remains a need to determine the effectiveness of IABP use in this population. In the absence of definitive evidence to guide the use of IABP in high risk PCI, data from observational studies can provide key insights into current practice and effectiveness of IABP use in patients undergoing high risk PCI. Accordingly, we analyzed data from the National Cardiovascular Data Registry (NCDR®) CathPCI Registry® to examine the use and outcomes associated with IABP among patients undergoing high risk PCI.
Methods
Data Source
The CathPCI Registry has been described previously15. In brief, the CathPCI Registry is a national, voluntary cardiac catheterization laboratory registry that catalogs the clinical data and outcomes of both cardiac catheterization and PCI at more than 600 hospitals across the United States. The registry includes a standardized set of data elements and definitions, systematic data entry and transmission procedures, and rigorous data quality assurance standards. The complete definitions of all variables were prospectively defined by a committee of the ACC and are available at the ACC Web site (http://www.acc.org/ncdr/cathlab.htm). Data are collected retrospectively or concurrently and represent consecutive patients treated at each institution and submitted to the CathPCI Registry. All data undergoes extensive quality checks and a random sample of CathPCI sites undergo external auditing to further ensure data quality.
Patient Population
To examine patterns of IABP use and to determine its association with outcomes, we identified a cohort of admissions for patients undergoing high risk PCI at a participating hospital between January 1, 2005 and December 31, 2007 (n=192,716). PCI was considered to be high risk if at least one of the following features was present: unprotected left main artery as the target vessel, cardiogenic shock, severely depressed left ventricular function (<30%), or ST segment elevation myocardial infarction 14. For patients with more than one PCI during a single hospital admission, we excluded data from the additional PCI (n=9,230). In addition, we excluded PCIs in which it could not be determined whether an IABP was inserted (n=5), PCIs performed on patients with severe aortic insufficiency (n=3360), and PCIs performed at hospitals that averaged fewer than 10 high risk PCIs per year (n=1170). A total of 181,599 high risk patients who underwent PCI at 681 hospitals were available for analysis.
Outcomes
The primary outcome of the study was in-hospital mortality. Secondary outcomes of interest included vascular complications - bleeding (drop in hemoglobin >3g/dl or hematoma >10cm), access artery occlusion (total obstruction of the vessel requiring surgical repair), pseudoaneurysm, dissection, and embolism.
Data Analysis
Using the cohort defined above, we examined the proportion of high risk PCI patients who received an IABP across NCDR hospitals. We compared demographics, cardiac status, comorbid conditions, and cardiac anatomy between patients who did and did not have an IABP inserted during high risk PCI. A Chi-square test was used for categorical variables, and an F-test from ANOVA was used for continuous variables. In order to identify the factors most strongly associated with IABP placement, we developed a logistic regression model with the receipt of an IABP as the dependent variable. Candidate variables were identified on the basis of clinical sensibility and review of the literature, and selected for inclusion in the final model using stepwise selection (entry P≤0.15, retention P≤0.05). Multicollinearity was evaluated through examination of Pearson Correlation Coefficients. Variables included in the final model included demographics (age, gender, race, and payor), cardiac status (admitting symptoms, cardiogenic shock, presence of heart failure, New York Heart Association class, left ventricular ejection fraction, and results of non-invasive testing), past medical history (prior MI, history of valve surgery, diabetes, renal dysfunction, renal dialysis, peripheral vascular disease, hypertension, tobacco use, chronic lung disease, dyslipidemia, family history of coronary artery disease, and prior PCI or coronary artery bypass grafting), and procedural characteristics (PCI status, door to balloon time). To assess the extent to which variation in the use of IABP was explained by clustering at the site level (ie by differences across hospitals), we developed a hierarchical logistic regression model using the variables identified previously and calculated a hospital-specific median odds ratio (OR)16, 17. The median OR represents that odds that identical patient at randomly chosen hospitals would receive an IABP during high risk PCI.
We then assessed the association of IABP use with outcomes. To accomplish this, we categorized hospitals by their proportional use of IABP in high risk PCI and grouped them into corresponding quartiles. We compared sociodemographics, past medical history, and cardiac status, and past medical history of patients treated at hospitals with high and low proportional IABP use. We also compared hospitals characteristics including profit type, number of beds, teaching status, census region, and annual volume of high risk PCI across hospital quartiles. We performed multivariable hierarchical logistic regression with in-hospital mortality as the dependent variable, after accounting for patient characteristics and hospital-level variables significantly associated IABP use identified in the previous model. In order to examine the relative contribution of each class of variables to the model, we repeated the hierarchical logistic regression models in a sequential fashion by incrementally introducing demographics, cardiac status, past medical history, and hospital characteristics into the models.
We further assessed the association of IABP with outcomes within subgroups of high risk PCI. For each subgroup, we performed multivariable hierarchical logistic regression analysis to determine the independent association of IABP use and mortality. Additional sensitivity analyses were performed excluding patients whose PCI was characterized as salvage, and again excluding patients with peripheral vascular disease in whom IABP insertion may not have been possible. Finally, adjusted analyses were repeated to assess the independent association of differences in IABP with vascular complications including hematoma, access site occlusion, peripheral embolization, dissection, pseudoaneurym, and arteriovenous fistula. All analyses were performed using SAS 9.1 (Cary, NC). The Yale Human Investigation Committee approved the analysis and determined that informed consent was not applicable to the data collected by the registry.
Results
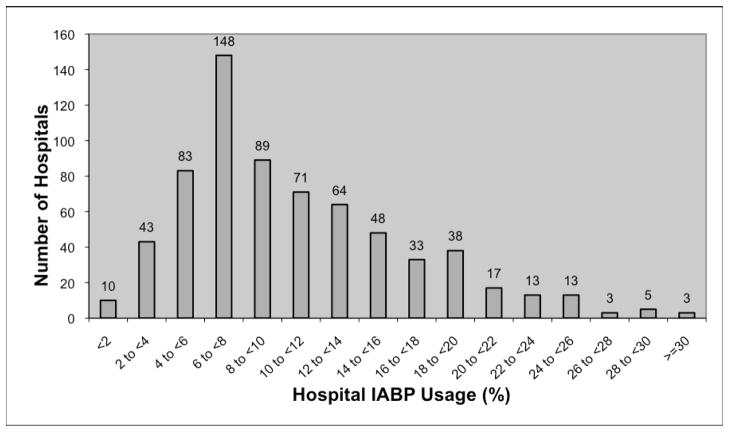
Among 181,599 high risk PCI performed at 681 hospitals, 21,259 (11.7%) had cardiogenic shock, 144,190 (79.4%) presented with STEMI, 3,592 (2.0%) underwent unprotected left main PCI, and 37,394 (20.6%) had an LVEF <30% (Patients could have more than one high risk characteristic). An IABP was used in 44.4% of cardiogenic shock patients, 10.3% of patients presenting with STEMI, 28.1% of patients undergoing PCI of an unprotected left main artery, and 13.9% of patients with LVEF ≤30%. The volumes of high risk PCI and the use of IABP varied significantly across participating hospitals. The median number of high risk cases was 202 (Range: 30–1337; Interquartile Range (IQR): 102 to 340), and the median number of IABP cases was 19 (Range: 0 to 223; IQR 9 to 36). Overall, IABPs were used in 10.5% of high risk cases, and IABP use increased modestly over the study period from 10.2% in the first quarter of 2005 to 11.5% in fourth quarter of 2007. There was substantial variation in proportional IABP use across hospitals (Figure 1).
Figure 1.
Hospital IABP Usage
Patients who received an IABP were a higher risk population than patients without an IABP (Table 1). Notably, IABP patients were older, more likely to have had renal insufficiency, present with non-STEMI, NYHA Class IV heart failure, or cardiogenic shock. There were statistically significant but clinically modest differences between the treating hospital characteristics of patients who did and did not receive an IABP. In multivariable analysis, the factors that were strongly associated with IABP use included cardiogenic shock, left ventricular ejection fraction, non-STEMI, heart failure on admission, and admission status. The model had a high c-statistic (0.82), but the high Hosmer-Lemeshow chi-square of 181 (p<0.001) suggests poor model calibration. In the hierarchical model, the median OR for the hospital effect was 1.93, suggesting a 93% greater odds of patients with identical covariates receiving an IABP at one randomly selected hospital compared with another (Table 2). As expected, the magnitude of the median OR is lower than certain clinical variables such as cardiogenic shock and LVEF. However, it does suggest that the hospital effect is exerting a significant influence on the decision of whether or not to use an IABP.
Table 1.
Select Baseline Characteristics of High Risk PCI Patients with and without an IABP
| Use of IABP | ||||
|---|---|---|---|---|
| Total (n=181599) | No (n=162609) | Yes (n=18990) | Overall P | |
|
| ||||
| Demographics | ||||
| Admission Status (%) | <0.001 | |||
| Outpatient Referral | 18549 (10.2) | 17628 (10.8) | 921 (4.8) | |
| Emergency Department | 96382 (53.0) | 85474 (52.6) | 10854 (57.2) | |
| Transfer from Acute Care Facility | 62602 (34.5) | 55887 (34.4) | 6715 (35.4) | |
| Transfer from Non-Acute Care Facility | 2099 (1.2) | 1885 (1.2) | 214 (1.1) | |
| Other | 2021 (1.1) | 1735 (1.1) | 286 (1.5) | |
| Age: Mean (SD) | 61.9 (13.1) | 61.7 (13.1) | 63.7 (13.0) | <0.001 |
| Female (%) | 52491 (28.9) | 46723 (28.7) | 5768 (30.4) | <0.001 |
| Race (%) | <0.001 | |||
| White | 153097 (84.3) | 137197 (84.4) | 15900 (83.7) | |
| Black | 11643 (6.4) | 10600 (6.5) | 1043 (5.5) | |
| Other | 16859 (9.3) | 14812 (9.1) | 2047 (10.8) | |
| Insurance Payor (%) | <0.001 | |||
| Government | 82549 (45.5) | 73119 (45.0) | 9430 (49.7) | |
| Commercial | 56831 (31.3) | 51469 (31.7) | 5362 (28.2) | |
| HMO | 22283 (12.3) | 20038 (12.3) | 2245 (11.8) | |
| Other | 19936 (11.0) | 17983 (11.1) | 1953 (10.3) | |
| Cardiac Status | ||||
| Admission Sx Presentation (%) | <0.001 | |||
| No Angina | 6804 (3.7) | 6356 (3.9) | 448 (2.4) | |
| Atypical Chest Pain | 2225 (1.2) | 2124 (1.3) | 101 (0.5) | |
| Stable Angina | 4249 (2.3) | 4118 (2.5) | 131 (0.7) | |
| ACS: Unstable Angina | 11874 (6.5) | 11047 (6.8) | 827 (4.4) | |
| ACS: Non-ST Elevation MI | 12257 (6.7) | 9670 (5.9) | 2587 (13.6) | |
| ACS: ST Elevation MI | 144190 (79.4) | 129924 (79.5) | 14896 (78.4) | |
| Cardiogenic Shock (%) | 21259 (11.7) | 11818 (7.3) | 9441 (49.7) | <0.001 |
| Door to Balloon time (minutes): Mean (SD) | 107.6 (246.1) | 108.5 (253.6) | 101.2 (176.6) | 0.002 |
| CHF – Current Status (%) | 28794 (15.9) | 22774 (14.0) | 6020 (31.7) | <0.001 |
| PCI Status | <0.001 | |||
| Elective | 20189 (11.1) | 19496 (12.0) | 693 (3.6) | |
| Emergency | 120102 (66.1) | 105325 (64.8) | 6409 (77.8) | |
| Salvage | 2452 (1.4) | 1274 (0.8) | 1178 (6.2) | |
| Ejection Fraction: Mean (SD) | 41.4 (15.2) | 42.2 (15.1) | 33.9 (14.2) | <0.001 |
| History and Risk Factors | ||||
| Previous MI (>7 Days) | 42701 (23.5) | 38350 (23.6) | 4351 (22.9) | 0.039 |
| CHF – Previous History | 21127 (11.6) | 18669 (11.5) | 2458 (12.9) | <0.001 |
| Previous Valvular Surgery | 1480 (0.8) | 1284 (0.8) | 196 (1.0) | <0.001 |
| Diabetes | 46577 (25.6) | 41050 (25.2) | 5527 (29.1) | <0.001 |
| Renal Failure – Previous History | 9250 (5.1) | 7768 (4.8) | 1482 (7.8) | <0.001 |
| Renal Failure – Dialysis | 2800 (1.5) | 2423 (1.5) | 377 (2.0) | <0.001 |
| Peripheral Vascular Disease | 16241 (8.9) | 14545 (8.9) | 1696 (8.9) | 0.95 |
| Hypertension | 116278 (64.0) | 104523 (64.3) | 11755 (61.9) | <0.001 |
| Current Use of Tobacco | 73587 (40.5) | 66786 (41.1) | 6801 (35.8) | <0.001 |
| Chronic Lung Disease | 27052 (14.9) | 23930 (14.7) | 3122 (16.4) | <0.001 |
| Dyslipidemia | 111215 (61.2) | 100773 (61.9) | 10482 (55.2) | <0.001 |
| Family History of CAD age <55 | 41704 (23.0) | 37865 (23.3) | 3839 (20.2) | <0.001 |
| Previous PCI | 39785 (21.9) | 36101 (22.2) | 3684 (19.4) | <0.001 |
| Previous CABG | 18812 (10.4) | 17274 (10.6) | 1538 (8.1) | <0.001 |
| Hospital Characteristics | ||||
| Hospital Location | <0.001 | |||
| Rural | 2289 (1.3) | 2085 (1.3) | 204 (1.1) | |
| Suburban | 163203 (89.9) | 146408 (90.0) | 16795 (88.4) | |
| Urban | 16107 (8.9) | 14116 (8.7) | 1991 (10.5) | |
| Hospital Profit Type | 0.237 | |||
| Government | 24276 (13.4) | 21705 (13.3) | 2571 (13.5) | |
| Private/Community | 47866 (26.4) | 42956 (26.4) | 4910 (25.9) | |
| University | 109457 (60.3) | 97948 (60.2) | 11509 (60.6) | |
| Patient Beds: Mean (SD) | 462 (232) | 461 (232) | 467 (238) | <0.001 |
ACS- Acute Coronary Syndrome; CAD- Coronary Artery Bypass Grafting; CABG- Coronary Artery Bypass Grafting; CHF- Congestive
Table 2.
Patient and Hospital Characteristics Associated with IABP Use (HGLM)
| Description | OR (95% CI) | P |
|---|---|---|
| Admission Status | ||
| Emergency Department | Reference | |
| Outpatient Referral | 0.83(0.76–0.91) | <0.001 |
| Transfer from Acute Care Facility | 1.03 (0.98–1.07) | 0.229 |
| Transfer from Non-Acute Care Facility | 0.96 (0.81–1.15) | 0.676 |
| Other | 1.12 (0.96–1.32) | 0.155 |
| Age | ||
| <50 | Reference | |
| 50 to <60 | 1.06 (1.00–1.12) | 0.057 |
| 60 to <70 | 1.16 (1.09–1.24) | <0.001 |
| 70 to <80 | 1.17 (1.08–1.25) | <0.001 |
| ≥80 | 0.98 (0.90–1.06) | 0.655 |
| Female | 0.87 (0.84–0.91) | <0.001 |
| Race | ||
| White | Reference | |
| Black | 0.83 (0.76–0.90) | <0.001 |
| Other | 1.11 (1.03–1.18) | 0.003 |
| Payor | ||
| Governmental | Reference | |
| Commercial | 1.02 (0.97–1.08) | 0.371 |
| HMO | 0.96 (0.90–1.02) | 0.216 |
| Other | 0.93 (0.87–1.00) | 0.039 |
| Without STEMI | 0.84 (0.78–0.90) | <0.001 |
| Cardiogenic Shock | 12.17 (11.66–12.71) | <0.001 |
| Door to Balloon Time | ||
| <90 minutes | 0.98 (0.92–1.04) | 0.458 |
| 90–120 minutes | 0.98 (0.91–1.06) | 0.599 |
| >120 minutes | Reference | |
| Missing | 0.91 (0.84–0.98) | 0.011 |
| CHF – Current Status | 1.74 (1.66–1.83) | <0.001 |
| PCI Status | ||
| Elective | Reference | |
| Urgent | 1.96 (1.77–2.17) | <0.001 |
| Emergency | 4.91 (4.43–5.44) | <0.001 |
| Salvage | 8.85 (6.67–10.21) | <0.001 |
| NYHA Classification | ||
| Class I | 0.75 (0.71–0.80) | <0.001 |
| Class II | 0.74 (0.68–0.80) | <0.001 |
| Class III | 0.79 (0.75–0.83) | <0.001 |
| Class IV | Reference | |
| Left Ventricular Wall Motion | ||
| Abnormal | Reference | |
| Not Measured | 1.10 (1.00–1.21) | 0.053 |
| Normal | 0.78 (0.72–0.85) | <0.001 |
| Left Ventricular Ejection Fraction | ||
| >50 | Reference | |
| >40 to 50 | 1.46 (1.35–1.58) | <0.001 |
| >30 to 40 | 2.64 (2.45–2.86) | <0.001 |
| >20 to 30 | 5.15 (4.76–5.58) | <0.001 |
| >10 to 20 | 7.73 (7.06–8.45) | <0.001 |
| 1 to 10 | 12.53 (10.69–14.69) | <0.001 |
| Not measured | 2.80 (2.51–3.13) | <0.001 |
| Stress Test | ||
| Not reported | Reference | |
| Negative | 0.81 (0.72–0.90) | <0.001 |
| Equivocal | 0.98 (0.82–1.17) | 0.787 |
| Positive | 0.76 (0.70–0.84) | <0.001 |
| CHF – Previous History | 0.85 (0.80–0.91) | <0.001 |
| Previous Valvular Surgery | 1.18 (0.98–1.42) | 0.086 |
| Diabetes | 1.13 (1.09–1.18) | <0.001 |
| Peripheral Vascular Disease | 0.80 (0.74–0.85) | <0.001 |
| Hypertension | 0.91 (0.88–0.95) | <0.001 |
| Current Use of Tobacco | 0.82 (0.79–0.86) | <0.001 |
| Chronic Lung Disease | 0.95 (0.90–1.00) | 0.051 |
| Dyslipidemia | 0.92 (0.88–0.96) | <0.001 |
| Family History of CAD | 1.06 (1.01–1.11) | 0.022 |
| Previous PCI | 0.91 (0.87–0.95) | <0.001 |
| Previous CABG | 0.68 (0.63–0.73) | <0.001 |
| Hospital type | ||
| Government | 0.74 (0.42–1.28) | 0.280 |
| University | 0.84 (0.69–1.03) | 0.087 |
| Hospital-specific effects (Median OR) | 1.93 | |
Between Hospital Variation 0.47
Within Hospital Variation 1.02
Hospitals were grouped by proportional IABP use into quartiles as follows: Q1 (0.0% to 6.5%), Q2 (6.6% to 9.2%), Q3 (9.3% to 14.1%), and Q4 (14.2% to 40.0%). The characteristics of hospitals with a low proportion of IABP use were comparable to those of hospitals with a high proportion of IABP use (Table 3). However, patient characteristics varied significantly across the hospital quartiles such that patients treated at hospitals in Q4 were older, had lower LVEF, and a higher proportion had renal dysfunction, prior congestive heart failure, diabetes, prior coronary artery bypass grafting, and cardiogenic shock compared with patients treated at hospitals in Q1–Q3 (Table 3). Of note, a lower proportion of patients treated at Q4 hospitals presented with STEMI but a higher proportion underwent salvage PCI compared with Q1–Q3 hospitals. Unadjusted mortality in the study cohort was 4.9% and increased in a near linear fashion across hospital quartiles (Q1 4.2%, Q2 4.8%, Q3 5.1%, Q4 5.6%, p <0.001). The multivariable mortality had high c-statistic (0.89) but similar issues with model calibration (Hosmer-Lemeshow chi-square 86.9, p<0.001). After adjustment for differences in patient and hospital characteristics, in-hospital mortality did not vary across hospital quartiles (Table 4). Sequential introduction of variables into the model suggested that differences in mortality were to a large degree explained by differences in the cardiac status of patients undergoing high risk PCI. Differences in demographics, past medical history, and hospital characteristics had relatively little impact on the point estimates and associated confidence intervals. Similar findings were observed in subgroup analyses such that there was no subgroup in which a higher proportional use of IABP was associated with improved outcomes. Furthermore, findings were unchanged in the sensitivity analyses excluding patients undergoing salvage PCI and peripheral vascular disease.
Table 3.
Patients and Hospital Characteristics Stratified by Quartiles of Hospital IABP Usage
| Demographics | Total 181599 |
Hospital IABP Usage
|
Overall P | |||
|---|---|---|---|---|---|---|
| Quartile 1 41941 |
Quartile 2 49962 |
Quartile 3 50165 |
Quartile 4 39531 |
|||
| Admission Status | <0.001 | |||||
| Outpatient Referral | 18549 (10.2) | 5945 (14.2) | 4183 (8.4) | 4727 (9.4) | 3694 (9.3) | |
| Emergency Department | 96328 (53.0) | 21725 (51.8) | 26230 (52.5) | 25205 (50.2) | 23168 (58.6) | |
| Transfer from Acute Care Facility | 62602 (34.5) | 13298 (31.7) | 18562 (37.2) | 18949 (37.8) | 11793 (29.8) | |
| Transfer from Non-Acute Facility | 2099 (1.2) | 461 (1.1) | 560 (1.1) | 662 (1.3) | 416 (1.1) | |
| Other | 2021 (1.1) | 512 (1.2) | 427 (0.9) | 622 (1.2) | 460 (1.2) | |
| Age: Mean (SD) | 61.9 (13.1) | 61.7 (13.1) | 61.3 (13.0) | 62.3 (13.2) | 62.4 (13.1) | <0.001 |
| Female (%) | 52491 (28.9) | 12244 (29.2) | 14189 (28.4) | 14563 (29.0) | 11495 (29.1) | 0.031 |
| Race | <0.001 | |||||
| White (%) | 153097 (84.3) | 36236 (86.4) | 42781 (85.6) | 40844 (81.4) | 33236 (84.1) | |
| Black (%) | 11643 (6.4) | 2551 (6.1) | 3238 (6.5) | 3478 (6.9) | 2376 (6.0) | |
| Other (%) | 16859 (9.3) | 3154 (7.5) | 3943 (7.9) | 5843 (11.6) | 3919 (9.9) | |
| Payor | <0.001 | |||||
| Government (%) | 82549 (45.5) | 19747 (47.1) | 22430 (44.9) | 22024 (43.9) | 18348 (46.4) | |
| Commercial (%) | 56831 (31.3) | 13779 (32.9) | 15628 (31.3) | 15783 (31.5) | 11641 (29.4) | |
| HMO (%) | 22283 (12.3) | 3798 (9.1) | 5972 (12.0) | 7179 (14.3) | 5334 (13.5) | |
| Other (%) | 19936 (11.0) | 4617 (11.0) | 5932 (11.9) | 5179 (10.3) | 4208 (10.6) | |
| Cardiac Status | ||||||
| Admission Symptom Presentation | <0.001 | |||||
| No Angina (%) | 6804 (37) | 1492 (3.6) | 1541 (3.1) | 2081 (4.2) | 1690 (4.3) | |
| Atypical Chest Pain (%) | 2225 (1.2) | 469 (1.1) | 526 (1.1) | 712 (1.4) | 518 (1.3) | |
| Stable Angina (%) | 4249 (2.3) | 1057 (2.5) | 969 (1.9) | 1236 (2.5) | 987 (2.5) | |
| ACS: Unstable Angina (%) | 11874 (6.5) | 3042 (7.3) | 2936 (5.9) | 3264 (6.5) | 2632 (6.7) | |
| ACS: Non-ST Elevation MI (%) | 12257 (6.7) | 2641 (6.3) | 3024 (6.1) | 3516 (7.0) | 3076 (7.8) | |
| ACS: ST Elevation MI (%) | 144190 (79.4) | 33240 (79.3) | 40966 (82.0) | 39356 (78.5) | 30628 (77.5) | |
| Cardiogenic Shock (%) | 21259 (11.7) | 4122 (9.8) | 5479 (11.0) | 6204 (12.4) | 5454 (13.8) | <0.001 |
| Door to Balloon time: Mean (SD) | 107.6 (246.1) | 109.1 (256.5) | 108.7 (253.4) | 104.1 (238.6) | 109.1 (235.3) | 0.038 |
| CHF - Current Status (%) | 28794 (15.9) | 5897 (14.1) | 7600 (15.2) | 8595 (17.1) | 6702 (17.0) | <0.001 |
| PCI Status | <0.001 | |||||
| Elective | 20189 (11.1) | 4446 (10.6) | 5149 (10.3) | 5908 (11.8) | 4686 (11.9) | |
| Urgent | 38824 (21.4) | 9651 (23.0) | 10706 (21.4) | 10623 (21.2) | 7844 (19.8) | |
| Emergency | 120102 (66.1) | 27351 (65.2) | 33521 (67.1) | 32887 (65.6) | 26343 (66.6) | |
| Salvage | 2452 (1.4) | 485 (1.2) | 582 (1.2) | 740 (1.5) | 645 (1.6) | |
| NYHA | <0.001 | |||||
| Class I | 40485 (22.3) | 10592 (25.3) | 10137 (20.3) | 11320 (22.6) | 8436 (21.3) | |
| Class II | 16279 (9.0) | 3879 (9.2) | 4384 (8.8) | 4136 (8.2) | 3880 (9.8) | |
| Class III | 36896 (20.3) | 8955 (21.4) | 10622 (21.3) | 9656 (19.2) | 7663 (19.4) | |
| Class IV | 87939 (48.4) | 18515 (44.1) | 24819 (49.7) | 25053 (49.9) | 19552 (49.5) | |
| Ejection Fraction: Mean (SD) | 41.4 (15.2) | 41.3 (15.1) | 42.4 (15.0) | 41.2 (15.5) | 40.6 (15.2) | <0.001 |
| History and Risk Factors | ||||||
| Previous MI (>7 Days) | 42701 (23.5) | 10252 (24.4) | 11296 (22.6) | 11572 (23.1) | 9581 (24.2) | <0.001 |
| CHF - Previous History | 21127 (11.6) | 4779 (11.4) | 5128 (10.3) | 6211 (12.4) | 5009 (12.7) | <0.001 |
| Previous Valvular Surgery | 1480 (0.8) | 315 (0.8) | 306 (0.6) | 438 (0.9) | 421(1.1) | <0.001 |
| Diabetes | 46577 (25.7) | 10538 (25.1) | 12492 (25.0) | 13057 (26.0) | 10490 (26.5) | <0.001 |
| Renal Failure - Previous History | 9250 (5.1) | 1900 (4.5) | 2343 (4.7) | 2755 (5.5) | 2252 (5.7) | <0.001 |
| Renal Failure - Dialysis | 2800 (1.5) | 573 (1.4) | 670 (1.3) | 888 (1.8) | 669 (1.7) | <0.001 |
| Peripheral Vascular Disease | 16241 (8.9) | 3713 (8.9) | 4353 (8.7) | 4537 (9.0) | 3638 (9.2) | 0.055 |
| Hypertension | 116278 (64.0) | 26818 (63.9) | 31587 (63.2) | 32363 (64.5) | 25510 (64.5) | <0.001 |
| Current Use of Tobacco | 73587 (40.5) | 17648 (42.1) | 21387 (42.8) | 19178 (38.2) | 15374 (38.9) | <0.001 |
| Chronic Lung Disease | 27052 (14.9) | 6058 (14.4) | 7619 (15.2) | 7349 (14.6) | 6026 (15.2) | <0.001 |
| Dyslipidemia | 111215 (61.2) | 26555 (63.3) | 30103 (60.3) | 30584 (61.0) | 23973 (60.6) | <0.001 |
| Family History of CAD age <55 | 41704 (23.0) | 9645 (23.0) | 11577 (23.2) | 11132 (22.2) | 9350 (23.7) | <0.001 |
| Previous PCI | 39785 (21.9) | 9471 (22.6) | 10703 (21.4) | 11000 (21.9) | 8611 (21.8) | <0.001 |
| Previous CABG | 18812 (10.4) | 4415 (10.5) | 4837 (9.7) | 5278 (10.5) | 4282 (10.8) | <0.001 |
| Hospital Characteristics | ||||||
| Location | <0.001 | |||||
| Rural | 2289 (1.3) | 838 (2.0) | 315 (0.6) | 746(1.5) | 390 (1.0) | |
| Suburban | 163203 (89.9) | 39304 (93.7) | 44854 (89.8) | 44679 (89.1) | 34366 (86.9) | |
| Urban | 16107 (8.9) | 1799 (4.3) | 4793 (9.6) | 4740 (9.4) | 4775 (12.1) | |
| Profit Type | <0.001 | |||||
| Government | 24276 (13.4) | 5520 (13.2) | 7338 (14.7) | 5737 (11.4) | 5681 (14.4) | |
| Private/Community | 47866 (26.4) | 12057 (28.8) | 12196 (24.4) | 12705 (25.3) | 10908 (27.6) | |
| University | 109457 (60.3) | 24364 (58.1) | 30428 (60.9) | 31723 (63.2) | 22942 (58.0) | |
| Patient Beds: Mean (SD) | 461.9 (232.2) | 429.8 (231.8) | 457.0 (184.0) | 485.6 (257.2) | 472.0 (249.1) | <0.001 |
Table 4.
Hospital IABP Volume and In-Hospital Mortality*
| Hospital IABP Usage
|
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|
| |||||
| Overall | Unadjusted | Ref | 1.17 (1.07–1.28) | 1.26 (1.16–1.37) | 1.36 (1.25–1.49) |
| Adjustment: (1) | Ref | 1.18 (1.08–1.28) | 1.22 (1.12–1.33) | 1.32 (1.21–1.44) | |
| Adjustment: (2) | Ref | 1.10 (0.99–1.23) | 1.04 (0.93–1.16) | 1.07 (0.95–1.20) | |
| Adjustment: (3) | Ref | 1.10 (0.98–1.23) | 1.03 (0.92–1.15) | 1.06 (0.94–1.19) | |
| Adjustment: (4) | Ref | 1.11 (0.99–1.24) | 1.03 (0.92–1.15) | 1.06 (0.94–1.18) | |
|
| |||||
| Subgroups: | |||||
|
| |||||
| STEMI | Adjustment: (4) | Ref | 1.09 (0.96–1.23) | 1.01 (0.90–1.14) | 1.02 (0.90–1.15) |
| LVEF <30% | Adjustment: (4) | Ref | 1.29 (1.09–1.54) | 1.19 (1.00–1.42) | 1.13 (0.94–1.35) |
| Unprotected Left Main | Adjustment: (4) | Ref | 0.83 (0.53–1.32) | 0.92 (0.59–1.42) | 0.95 (0.60–1.50) |
| Cardiogenic Shock | Adjustment: (4) | Ref | 1.19 (1.03–1.38) | 0.99 (0.86–1.14) | 1.06 (0.91–1.22) |
- Demographics
- Demographics and Cardiac Status
- Demographics, Cardiac Status, Medical History, PCI Status
- Demographics, Cardiac Status, Medical History, PCI Status, and Hospital Characteristics
Unadjusted complication rates differed significantly across hospital quartiles (Table 5). Crude rates of vascular complications were highest at Q4 hospitals (Unadjusted OR: Q1: reference; Q2: OR 1.01 (0.86–1.19); Q3: OR 1.25 (1.07–1.47); Q4 1.22 (1.04–1.43)). However, after multivariable adjustment, the risk of vascular complications were similar across hospital quartiles (Q1: reference; Q2: OR 0.96 (.81–1.23); Q3: OR 1.16 (0.99–1.36); Q4: OR 1.11 (0.94–1.31).
Table 5.
Adverse Outcomes and Complications Stratified by Quartiles of Hospital IABP Usage
| Total 181599 |
Hospital IABP Usage
|
P | ||||
|---|---|---|---|---|---|---|
| Quartile 1 41941 |
Quartile 2 49962 |
Quartile 3 50165 |
Quartile 4 39531 |
|||
| In-hospital death | 8944 (4.9) | 1746 (4.2) | 2414 (4.8) | 2577 (5.1) | 2207 (5.6) | <0.001 |
|
Vascular Complications (%)
| ||||||
| Bleeding | 8637 (4.8) | 1789 (4.3) | 2110 (4.2) | 2732 (5.4) | 2006 (5.1) | <0.001 |
| Access Site Occlusion | 150 (0.1) | 26 (0.1) | 31 (0.1) | 36 (0.1) | 57 (0.1) | <0.001 |
| Peripheral Embolization | 234 (0.1) | 40 (0.1) | 61 (0.1) | 63 (0.1) | 70 (0.2) | 0.012 |
| Dissection | 577 (0.3) | 85 (0.2) | 108 (0.2) | 268 (0.5) | 116 (0.3) | <0.001 |
| Pseudoaneurysm | 816 (0.4) | 133 (0.3) | 216 (0.4) | 238 (0.5) | 229 (0.6) | <0.001 |
| Vascular-AV Fistula | 128 (0.1) | 26 (0.1) | 31 (0.1) | 36 (0.1) | 35 (0.1) | 0.428 |
IABP- Intra-aortic Balloon Pump
Discussion
In this cohort of high risk PCI patients, IABP use varied significantly across hospitals. However, the observed variation in IABP use was not associated with differences in either inhospital mortality or complication rates across hospitals. The outcomes of patients treated at hospitals using IABP more selectively were comparable to those of patients treated at hospitals that used IABP more frequently. Our findings provide no evidence to support the greater use of IABP at some hospitals and indicate a pressing need to further define the settings where this intervention provides a net benefit.
Data from the Benchmark Registry suggest that a fifth of all IABP insertions occur in the setting of PCI, and that half of those are performed to provide support and stabilization to patients who are not in cardiogenic shock18. In the present analysis, IABPs were used in slightly less than 10% of high risk PCI, and hospital use varied such that there was at least a two-fold difference in IABP use between hospital quartiles that used IABP more and less frequently. Similar variation has been demonstrated in rates of IABP use during coronary artery bypass grafting19. These findings highlight the fact that much of IABP use is discretionary. The decision to insert an IABP is likely influenced by physician training, clinical experience, and local practice patterns rather than high quality evidence from clinical studies. To date, however, these favorable properties of IABP have not been convincingly linked to improvements in patient outcome.
The evidence that supports IABP for high risk PCI comes from case series 20–22, and retrospective analyses 2, 4–6, 13. Although these studies demonstrated feasibility and suggested efficacy of routine IABP use, their findings have not been supported by data from randomized trials. In randomized trials that enrolled patients with AMI undergoing primary PCI, routine IABP use was not associated with differences in procedural success or clinical outcomes 8, 9, 11, 12. Collectively, these studies do not support the routine use of IABP for patients at high risk of adverse outcomes, but leaves open the question of how aggressive we should be in using this technology.
Several factors have likely contributed to the gap between practice and evidence. First, recruiting patients into randomized trials of IABP has proven difficult. For example, two trials examining the effect of IABP patients with cardiogenic shock failed to meet target enrollment 23. Second, advances in technology, particularly the routine use of coronary stents, have improved procedural success and significantly reduced risks associated with PCI 24. As procedural risks have declined, identifying patients who are at sufficiently high risk of hemodynamic compromise that an IABP would be useful becomes increasingly difficult. Third, in the absence of definitive evidence, physicians and institutions have developed individual thresholds for IABP use. Once practitioners have established a routine use of a new technology, the perceived need to formally evaluate its efficacy may diminish.
Our data cannot be used to define the precise threshold at which an IABP would be beneficial. There are patients, notably those who present with or develop cardiogenic shock refractory to volume expansion, who likely benefit from IABP use, and studies suggest that IABPs have been persistently underused in this situation 1, 25. Instead, the results of this analysis highlight the variations in IABP use that exist across hospitals, and should prompt interventional cardiologists and PCI capable hospitals to critically examine their practice patterns and, perhaps, consider adopting a more selective approach to IABP use in high risk PCI.
There are several important limitations to this analysis. First, this is a retrospective analysis of registry data. As such, we cannot exclude the possibility that unmeasured factors may confound our results. For example, although the NCDR contains more than 120 fields including the presence of cardiogenic shock, it does not directly capture potentially important variables such as baseline heart rate or blood pressure. Second, we did not perform a formal power calculation to determine whether we had enough power to detect significant differences in mortality across hospitals performing high risk PCI. Nevertheless, with a cohort of more than 180,000 high risk PCI and an overall mortality rate of 4.9%, we believe we could detect clinically meaningful differences across hospitals. Third, the observed variation in hospital use of IABP may not be due to difference in practice patterns, but may instead be a marker for differences between hospitals such as the skill of the interventional cardiologist, the quality of both intra and post-procedural care, or case mix. If so, higher rates of IABP use may mask significant differences in patient outcomes that would otherwise be measurable. Finally, our analysis did not address the timing of IABP use in relation to the performance of PCI, and this may be an important mediator of the benefits of discretionary IABP use.
In conclusion, among hospitals that participate in the NCDR, there was significant variation in the rate of IABP during and after high risk PCI. However, we found no evidence that the outcomes of patients treated at hospitals that used IABP more frequently were better than those of patients treated at hospitals that used IABP less frequently. These findings should prompt a reevaluation of the threshold for IABP in this population.
Acknowledgments
Funding Sources
This research was supported by the American College of Cardiology Foundation’s NCDR (National Cardiovascular Data Registry) and Datascope, a maker of IABPs. The views expressed in this manuscript represent those of the authors and do not necessarily represent the official views of the NCDR, its associated professional societies identified at www.ncdr.com or Datascope.
CathPCI Registry® is an initiative of the American College of Cardiology Foundation and The Society for Cardiovascular Angiography and Interventions.
The project described was also supported by Award Number U01HL105270 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Footnotes
Disclosures
Dr. Curtis and Mr. Wang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Krumholz discloses that he chairs a Cardiac Scientific Advisory Board for UnitedHealth.
References
- 1.Chen EW, Canto JG, Parsons LS, Peterson ED, Littrell KA, Every NR, Gibson CM, Hochman JS, Ohman EM, Cheeks M, Barron HV. Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock. Circulation. 2003;108:951–957. doi: 10.1161/01.CIR.0000085068.59734.E4. [DOI] [PubMed] [Google Scholar]
- 2.Brodie BR, Stuckey TD, Hansen C, Muncy D. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarction. Am J Cardiol. 1999;84:18–23. doi: 10.1016/s0002-9149(99)00185-x. [DOI] [PubMed] [Google Scholar]
- 3.Ohman EM, George BS, White CJ, Kern MJ, Gurbel PA, Freedman RJ, Lundergan C, Hartmann JR, Talley JD, Frey MJ. Use of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Results of a randomized trial. The Randomized IABP Study Group. Circulation. 1994;90:792–799. doi: 10.1161/01.cir.90.2.792. [DOI] [PubMed] [Google Scholar]
- 4.Briguori C, Sarais C, Pagnotta P, Airoldi F, Liistro F, Sgura F, Spanos V, Carlino M, Montorfano M, Di Mario C, Colombo A. Elective versus provisional intra-aortic balloon pumping in high-risk percutaneous transluminal coronary angioplasty. Am Heart J. 2003;145:700–707. doi: 10.1067/mhj.2003.14. [DOI] [PubMed] [Google Scholar]
- 5.Briguori C, Airoldi F, Chieffo A, Montorfano M, Carlino M, Sangiorgi GM, Morici N, Michev I, Iakovou I, Biondi-Zoccai G, Colombo A. Elective versus provisional intraaortic balloon pumping in unprotected left main stenting. Am Heart J. 2006;152:565–572. doi: 10.1016/j.ahj.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Mishra S, Chu WW, Torguson R, Wolfram R, Deible R, Suddath WO, Pichard AD, Satler LF, Kent KM, Waksman R. Role of prophylactic intra-aortic balloon pump in high-risk patients undergoing percutaneous coronary intervention. Am J Cardiol. 2006;98:608–612. doi: 10.1016/j.amjcard.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Barron HV, Every NR, Parsons LS, Angeja B, Goldberg RJ, Gore JM, Chou TM. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001;141:933–939. doi: 10.1067/mhj.2001.115295. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Marsalese D, Brodie BR, Griffin JJ, Donohue B, Costantini C, Balestrini C, Wharton T, Esente P, Spain M, Moses J, Nobuyoshi M, Ayres M, Jones D, Mason D, Grines L, O’Neill WW, Grines CL. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial Investigators. J Am Coll Cardiol. 1997;29:1459–1467. doi: 10.1016/s0735-1097(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 9.Redwood S. [Accessed May 6, 2010.];Balloon-pump Assisted Coronary Intervention Study. 2009 Sep 25; doi: 10.1016/j.ahj.2009.09.015. tctmd.com. Available at: http://www.tctmd.com/show.aspx?id=84124. [DOI] [PubMed]
- 10.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Jr, Koch KT, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, Ohman EM. Intra-aortic Balloon Counterpulsation and Infarct Size in Patients With Acute Anterior Myocardial Infarction Without Shock. JAMA: The Journal of the American Medical Association. 2011;306:1329–37. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 12.van ’t Hof AW, Liem AL, de Boer MJ, Hoorntje JC, Suryapranata H, Zijlstra F. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. Eur Heart J. 1999;20:659–665. doi: 10.1053/euhj.1998.1348. [DOI] [PubMed] [Google Scholar]
- 13.O’Murchu B, Foreman RD, Shaw RE, Brown DL, Peterson KL, Buchbinder M. Role of intraaortic balloon pump counterpulsation in high risk coronary rotational atherectomy. J Am Coll Cardiol. 1995;26:1270–1275. doi: 10.1016/0735-1097(96)81473-2. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:156–175. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 15.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 16.Larsen K, Merlo J. Appropriate Assessment of Neighborhood Effects on Individual Health: Integrating Random and Fixed Effects in Multilevel Logistic Regression. American Journal of Epidemiology. 2005;161:81–88. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 17.Larsen K, Petersen JH, Budtz-Jørgensen E, Endahl L. Interpreting Parameters in the Logistic Regression Model with Random Effects. Biometrics. 2000;56:909–914. doi: 10.1111/j.0006-341x.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson JJ, 3rd, Cohen M, Freedman RJ, Jr, Stone GW, Miller MF, Joseph DL, Ohman EM. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol. 2001;38:1456–1462. doi: 10.1016/s0735-1097(01)01553-4. [DOI] [PubMed] [Google Scholar]
- 19.Ghali WA, Ash AS, Hall RE, Moskowitz MA. Variation in hospital rates of intraaortic balloon pump use in coronary artery bypass operations. Ann Thorac Surg. 1999;67:441–445. doi: 10.1016/s0003-4975(98)01138-2. [DOI] [PubMed] [Google Scholar]
- 20.Kreidieh I, Davies DW, Lim R, Nathan AW, Dymond DS, Banim SO. High-risk coronary angioplasty with elective intra-aortic balloon pump support. Int J Cardiol. 1992;35:147–152. doi: 10.1016/0167-5273(92)90171-x. [DOI] [PubMed] [Google Scholar]
- 21.Kahn JK, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Hartzler GO. Supported “high risk” coronary angioplasty using intraaortic balloon pump counterpulsation. J Am Coll Cardiol. 1990;15:1151–1155. doi: 10.1016/0735-1097(90)90257-p. [DOI] [PubMed] [Google Scholar]
- 22.Voudris V, Marco J, Morice MC, Fajadet J, Royer T. “High-risk” percutaneous transluminal coronary angioplasty with preventive intra-aortic balloon counterpulsation. Cathet Cardiovasc Diagn. 1990;19:160–164. doi: 10.1002/ccd.1810190303. [DOI] [PubMed] [Google Scholar]
- 23.Ohman EM, Hochman JS. Aortic counterpulsation in acute myocardial infarction: physiologically important but does the patient benefit? Am Heart J. 2001;141:889–892. doi: 10.1067/mhj.2001.115296. [DOI] [PubMed] [Google Scholar]
- 24.Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes DR, Jacobs A, Kelsey SF, King SB, 3rd, Myler R, Slater J, Stanek V, Vlachos HA, Detre KM. Percutaneous coronary intervention in the current era compared with 1985–1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–2951. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 25.Menon P, Totaro P, Youhana A, Argano V. Reduced vascular complication after IABP insertion using smaller sized catheter and sheathless technique. Eur J Cardiothorac Surg. 2002;22:491–492. doi: 10.1016/s1010-7940(02)00345-7. author reply 492–493. [DOI] [PubMed] [Google Scholar]