Abstract
Objective
Few tests of functional motor behavior are useful for rapidly screening people for lower extremity peripheral neuropathy. The goal of this study was to improve the widely used Tandem Walking test (TW).
Methods
We tested adult normals and ambulatory peripheral neuropathy patients (PN) with eyes open and eyes closed, while they performed TW on industrial carpeting, in sock-covered feet. Each subject wore a torso-mounted inertial motion unit to measure kinematic data. PN subjects’ data were also compared to historical data on patients with vestibular impairments (VI).
Results
The normal and PN groups differed significantly on TW on the number of steps completed. PN and VI data also differed significantly on both visual conditions. Kinematic data showed that PN patients were more unstable than normals. For the number of steps taken during the eyes open condition receiver operating characteristic (ROC) values were only 0.81. For the number of steps taken during the eyes closed condition, however, ROC=0.88. Although not optimal, this ROC value is better. Sensitivity and specificity at a cut-off of 2 steps were 0.81 and 0.92, respectively, and at a cut-off of 3 steps was 0.86 and 0.75, respectively. ROC values for kinematic data were all < 0.8 and, when combined with the ROC value for the number of steps, the total ROC value did not improve appreciably.
Conclusions
Although not ideal for screening patients who may have peripheral neuropathy, counting the number of steps during TW is a quick and useful clinical test. TW is most sensitive to peripheral neuropathy patients when they are tested with eyes closed.
Keywords: balance testing, tandem gait, neurology testing, clinical examination, sensitivity and specificity
INTRODUCTION
Balance tests are often cited as being useful for screening people suspected of having vestibular impairments (1, 2) but these tests may also be useful for screening patients with neurologic impairments. (3–5) Computerized posturography systems that test standing balance are commercially available. Some patients with peripheral neuropathy are impaired on computerized dynamic posturography testing but not with the same pattern as patients with vestibular disorders. (6–8) Although some primary care physicians screen people for peripheral neuropathy using monofilament testing that kind of testing is designed to screen for only small fiber neuropathy. Balance testing may be sensitive to large fiber as well as small fiber neuropathy and may provide a more functional indicator of balance skills. The cost of a computerized balance testing system, and the size of the equipment, however, preclude using such systems in many clinics. (9) Therefore, a simple measure of walking balance may be useful for primary care physicians to augment their screening regimens, particularly for patients who may be at risk for falls.
Various versions of the tandem walking test (TW) (10, 11) have been in use for many years. (12) TW was originally developed for use on narrow rails with eyes open but eventually it became standardized with eyes closed. (11) Longridge and Mallinson recently reported that less than 30% of patients with vestibular disorders or normals could perform 5 steps of tandem walking with eyes closed. (13) Patients with vestibular impairments have impaired performance compared to normals. (14) Performance on several variations of TW declines slightly with age. (15–18) Despite its widespread use no studies have determined if TW actually distinguishes patients with peripheral neuropathies or sensory ataxia from normals.
To determine how people with peripheral neuropathies perform on this quick, inexpensive test we compared the scores of normal adults to patients with peripheral neuropathies. The usual measure of TW is the number of steps taken. To learn more about performance on TW we also complimented the number of steps by assessing trunk kinematics, measured with sophisticated instrumentation. Although we did not expect that clinicians would have this kind of sophisticated equipment planned to determine if more information about kinematics would be useful for understanding the qualitatively observable responses.
MATERIALS AND METHODS
Subjects
Participants included normal, control subjects (controls) and patients with lower extremity peripheral neuropathies (PN). Controls were recruited from among staff and visitors to our laboratory. Controls were screened with a brief health history and they were also tested with Dix-Hallpike maneuvers, head shaking in yaw rotations, and head impulse tests, to exclude vestibular disorders. (14) No subjects had had joint replacements, were missing any toes, had deformities of the toes, or had less than functional range of motion in all joints, and no subjects complained of pain while walking. All subjects were ambulatory without use of canes or other gait aids. All subjects wore comfortable clothes and performed all tests without shoes but, for good hygiene, all subjects wore socks. Subjects gave written informed consent prior to participation. This study was approved by the Institutional Review Board for Human Subjects Research for the corresponding author’s institution.
PN subjects were first identified from patient records. All PN subjects had already been diagnosed by board-certified neurologists with large (N=5), small (N=7) or mixed large and small fiber neuropathy (N=9), based on the neurologist’s clinical examination, which may have included electromyography testing. We were unable to determine the length of illness for PN subjects. None of them had developed their disease states within a month of testing. Details of inclusion/exclusion criteria are given in Table 1.
Table 1.
Table of inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Ambulate independently | Hip, knee or ankle joint replacements |
| Controls: no sensory losses, no balance complaints | Central neurologic damage |
| PN: Diagnosis of lower extremity peripheral neuropathy | Significant musculoskeletal limitations |
| Functional vision (at least 20/40) with corrective lenses | No use of walker or cane |
| Fluent speaking English | History of vestibular disorder |
| Indication of vestibular disorder based on screening | |
| No psychiatric diagnoses or cognitive limitations |
Instrumentation
During testing, each subject wore a lightweight vest with an inertial motion sensor (IMU; Xsens North America Inc., Los Angeles, CA), 5.25 X 3.75 X 2 cm, weight 28.3 g, centered on the back at the mid-thoracic level. The IMU was used to measure kinematic data, as described below.
Tests
The tests were administered by one of three technicians with 7 to 25 years of experience performing vestibular and balance testing. Technicians were unaware of subjects’ diagnoses. Inter-rater reliability for these tests has been established in tests of normals and patients with vestibular disorders. (14) For all measures inter-rater reliability was 0.94 to 0.99.
Subjects were asked to walk for 10 steps, heel-to-toe, without spaces between the steps. They performed Trial 1 with eyes open (EO). They performed Trial 2 with eyes closed (EC). The technicians recorded the maximum number of correct consecutive steps for a maximum of 10. Errors included taking a side step, making a space between the feet, and opening the eyes during the EC condition. Subjects were given one trial per condition, for a total of two trials. To avoid a learning effect repeated trials were not used. The EO condition was always given first. Staff members provided safety guarding during all tests. All tests were given in a quiet room with industrial carpeting.
Kinematic and behavioral analyses
After testing raw kinematic data were reduced by technical staff who had more than 15 years of experience working with kinematic data. They were blinded to the subjects’ groups. For kinematic analyses the following root mean square values of the IMU variables for the trunk segment were quantified and used for further analysis: resultant acceleration (TAR), angular velocity about the roll axis (TRV), angular velocity about the pitch axis (TPV), angular velocity about the yaw axis (TYV).
Statistical analyses
To describe differences in the dependent measures multilevel statistical techniques (19) were used, with a separate model fitted to each dependent variable. Within each model, within and between subjects effects were tested. Interaction effects were included in each model and tested. A likelihood ratio statistic that follows a chi-square distribution was used to compare changes over eye conditions between groups. Adjustments were made for multiple comparisons. Tests of the number of steps were adjusted for age. P <0.05 was considered as statistically significant.
Receiver Operating Characteristic (ROC) analysis is a type of statistical analyses that came out of signal detection theory (20). It is used in medical research to determine the discriminatory power of a clinical test. We subjected the dependent measures of the number of steps taken and the kinematic measures to ROC analyses. An ROC value of > 0.95 is considered excellent and means that the test has very high discriminative value; an ROC value of 0.50 is chance and means that the test has no discriminative value at all. When the ROC analysis is good to excellent then sensitivity to detecting patients and specificity to detecting normals can be calculated for different cut-points, i.e. different values of the test. To determine if any test is useful in identifying people with PN and to determine the optimal cut point on each test, we performed logistic regression and ROC analyses and provided corresponding sensitivity and specificity values for various cut offs. All statistical analyses were performed using SAS Statistical software, version 9.3 (SAS, Carry, NC).
RESULTS
The final sample included 61 controls and 21 PN subjects, described in Table 2. As indicated in the table PN subjects were significantly older than controls. PN subjects were recruited from patients seen in local hospitals staffed by neurologists at our medical school. They were tested between July 2009 and December 2010.
Table 2.
Demographic details of study sample. Mean age (yrs) (SD, range), number per gender.
| Group | Age | Females/Males |
|---|---|---|
| Normals | 49.6 (16.0, 23.3 to 77.0) | 30 F, 31 M |
| PN | 60 (12.4, 30.6 to 74.3) | 8F, 13 M |
PN subjects took significantly more consecutive steps with eyes open than eyes closed (p< 0.0001). Normals took significantly more steps than PN subjects in eyes open (p<0.0001) and eyes closed (p<0.0001) conditions. See Table 3. Of the 21 PN subjects only one subject slipped and had to be steadied by a staff member; another subject needed contact guarding on every step. No other subjects were touched by staff during test administration. To determine if these patients differed from other patients with balance disorders we also compared these data to data from 27 subjects with unilateral peripheral vestibular weakness from a previously published study on patients with vestibular impairments (VI) (14). The groups did not differ significantly by age. T-tests showed significantly fewer steps taken by PN than VI subjects on the eyes open, p< 0.01, and eyes closed conditions, p< 0.001. See Table 3.
Table 3.
Tandem walking. Number of consecutive steps per condition for normals, PN patients and VI patients. Adjusted means, (median, ranges). Age-adjusted tests showed that normals and PN subjects differed significantly for each condition, p < 0.0001.
| Eyes Open | Eyes Closed | |
|---|---|---|
| Normals | 9.25 (10, 3 to 10) | 5.76 (5, 1 to 10) |
| PN | 5.74 (4, 0 to 10) | 2.07 (1, 0 to 6) |
| VI | 7.8 (8, 4 to 10) | 3.8 (4, 0 to 10) |
On kinematic analyses PN subjects had significantly higher TRV, TPV and TYV, than normals, (p<0.001), and, regardless of group, subjects had significantly higher scores during eyes closed than eyes open conditions (p<0.001). For TAR the groups differed significantly (p=0.001) and the conditions differed significantly (p=0.0001). These results indicate that PN subjects showed greater instability in all planes than normals performing the tandem walk with eyes closed. See Figures 1 and 2.
Figure 1.
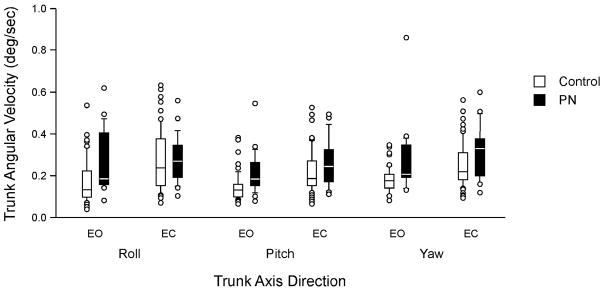
Trunk angular velocity by group and condition. EO, eyes open; EC, eyes closed. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, circles are outliers.
Figure 2.
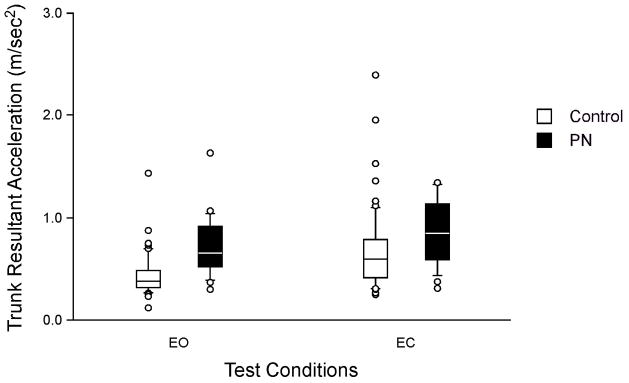
Trunk resultant acceleration by group and condition. EO, eyes open; EC, eyes closed. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, circles are outliers,
ROC analyses of the number of steps, comparing normals to PN subjects, were moderate with eyes open (ROC=0.81, 95% CI = 0.70 to 0.93) and stronger with eyes closed (ROC=0.88, 95% CI = 0.81 to 0.96). No clear cut-point for eyes open could be found; the best cut-point for eyes closed was 2 steps. See Table 4.
Table 4.
Tandem walking. ROC analyses for number of steps. Sensitivity and specificity at several scores normals and PN subjects
| Eyes open | Sensitivity (to PN subjects) | Specificity (to normals) |
|---|---|---|
| 3 steps | 0.43 | 0.98 |
| 5 steps | 0.62 | 0.95 |
| 7 steps | 0.67 | 0.89 |
| 8 steps | 0.67 | 0.84 |
| Eyes closed | ||
| 2 steps | 0.81 | 0.92 |
| 3 steps | 0.86 | 0.75 |
| 5 steps | 0.95 | 0.46 |
| 7 steps | 1.0 | 0.38 |
| 8 steps | 1.0 | 0.28 |
ROC analyses of the kinematic data for tests with eyes closed were calculated and then ROC values for kinematic data combined with the number of steps, for tests with eyes closed, were calculated. All ROC values for kinematic data, alone were low: TAR, ROC= 0.67; TYV, ROC=0.651; TPV, ROC=0.608; TRV, ROC= 0.561. When combined with the number of steps, for the eyes closed condition, ROC improved only very slightly: Steps + TAR, ROC=0.886; Steps + TYV, ROC= 0.882; Steps + TPV, ROC=0.885; Steps + TRV, ROC=0.888.
DISCUSSION
TW is widely known and used by physicians but it is still not well understood or validated. We have shown that on an easily observed measure of behavior, the number of steps taken, PN patients differed from controls. Many physicians routinely use the eyes open condition for tandem walking. The ROC analyses indicate, however, that, despite statistically significant differences between groups with eyes open, the eyes closed condition is the better measure. The sensitivity and specificity analyses showed that not many steps are needed for the test. The optimal cut-point was only 2 steps. Thus, even in a very small examining room, enough space should be available for this test.
Kinematic measures are not as easily quantified but are also important to elucidate the performance of PN patients. On all kinematic measures PN patients were more unstable than controls. The greater stability of controls probably allowed them to take more steps than PN patients, however combining the kinematic scores with the number of steps in the ROC analyses improved the ROC for the eyes closed condition only slightly, not enough to warrant collection of kinematic data during routine clinical screening.
Nevertheless the kinematic data from this study are useful for the physician. Some patients may have better innate motor skill than others. A patient might be able to perform more than 2 consecutive steps with eyes closed, using some unusual strategy, but that strategy might be reflected in abnormal kinematics. Therefore, if a physician observes that patient using an unusual movement pattern to perform the test, the physician should consider examining the problem further.
Patients with peripheral neuropathy have highly variable performance depending on the subtype of PN, disease stage, and other health conditions. Therefore this relatively small study should be considered as preliminary. Within the time period that we recruited PN subjects a larger sample was unavailable. Some patients declined to participate; other patients were excluded because they had joint replacements, were unable to walk unassisted (without gait aids), had additional neurologic diagnoses, or had significant otologic or musculoskeletal problems that might have confounded data interpretation. We standardized footwear by having subjects remove their shoes but wear socks, to maintain good hygiene. Clinicians who habitually test patients in their shoes or in bare feet should keep this difference in mind when performing TW. Future work should include larger samples and use groups that do not differ by age.
This study did not use patients with any known history of stroke or essential tremor. These conditions are relatively common in neurology practice and may be seen in patients who present with balance impairments. Therefore the primary care physician who screens such a patient should take these problems into account when interpreting data from balance testing.
We compared the data collected in this study to data collected from patients known to have vestibular disorders. The PN patients performed worse; also, patients with vestibular disorders typically complain of vertigo. Thus, this additional bit of information may help the primary care physician to determine the diagnosis or the need for specific specialty care referrals. These samples are relatively small, however, and both patient populations are highly variable. Therefore these findings should be considered preliminary.
The use of ROC and sensitivity/specificity analyses are important aspects of this study. Many reported tests show statistically significant differences between healthy and patient groups. Those differences may not be highly meaningful or important, however, if patients and controls are not sufficiently different to be distinguished on testing. Tests are only useful for screening if the scores of the groups are so different that the tests are sensitive to the patient population of interest and specific to normals. This concept is especially important when the health care provider who does the screening for PN, is a non-specialist, such as a physician who is not a neurologist, or a non-physician clinician such as a nurse or a therapist. At the cut-off of two steps TW with eyes closed has very good sensitivity and specificity. Ideally a test should have a high ROC value, and high sensitivity and specificity; with a larger sample those values might have been different. Thus, TW used in combination with the rest of the clinical examination should be useful indentifying those patients who might have peripheral neuropathy and who might benefit most from more detailed diagnostic testing.
Balance testing in primary care is important for identifying deficits that may suggest underlying illness and for predicting subsequent levels of functional decline and even mortality. (21, 22) In this regard, TW is valuable for the primary care physician because administering it takes only 30 seconds and requires no special equipment or extra space. It provides reliable data with which to facilitate further clinical decision-making, such as the need for more detailed examination, specific testing, or referral to a specialty service such as neurology. If the physician has a positive finding then referral to a specialty care physician, such as a neurologist, should be considered to obtain a definitive diagnosis. Diagnosis and treatment of balance disorders may improve patient functional abilities, quality of life and thus reduce health care costs. Use of TW, with eyes open for the patient to “get the idea” of the test, and with eyes closed for formal evaluation, may aid in achieving those goals.
Acknowledgments
We thank Christopher Miller of Wyle Integrated Science and Engineering Group, and the staff of the Center for Balance Disorders, Baylor College of Medicine for invaluable technical assistance.
Footnotes
Proprietary statement
No commercial or proprietary interests by any authors
Institutional approval
This study was approved by the Institutional Review Board for Human Subjects Research for Baylor College of Medicine and Affiliated Hospitals.
Financial disclosure
Supported by NIH grant R01DC009031 (HSC) and grants from the National Space Biomedical Research Institute through NASA NCC 9-58 (APM and JJB).
References
- 1.Hullar TE, Zee DS, Minor LB. Evaluation of the patient with dizziness. In: Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins KT, et al., editors. Cummings Otolaryngology Head and Neck Surgery. 3. Philadelphia: Mosby Elsevier; 2010. pp. 2304–27. [Google Scholar]
- 2.Mallinson AI, Longridge NS. Increasing the usefulness of tandem walking evaluation. J Otolaryngol Head Neck Surg. 2008;37:860–4. [PubMed] [Google Scholar]
- 3.Ropper AH, Samuels MA. Adams and Victor’s Principles of Neurology. 9. New York: McGraw-Hill; 2009. [Google Scholar]
- 4.Simon RP, Greenberg DA, Aminoff MJ. Clinical Neurology. 7. New York: McGraw-Hill; 2009. [Google Scholar]
- 5.Hoskovcová M, Ulmanová O, Sprdlík O, Sieger T, Nováková J, Jech R, et al. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum. 2013;12:27–34. doi: 10.1007/s12311-012-0384-4. [DOI] [PubMed] [Google Scholar]
- 6.Di Nardo W, Ghirlanda G, Cercone S, Pitocco D, Soponara C, Cosenza A, et al. The use of dynamic posturography to detect neurosensorial disorder in IDDM without clinical neuropathy. J Diabetes Complications. 1999;13(2):79–85. doi: 10.1016/s1056-8727(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 7.Emam AA, Gad AM, Ahmed MM, Assal HS, Mousa SG. Quantitative assessment of posture stability using computerised dynamic posturography in type 2 diabetic patients with neuropathy and its relation to glycaemic control. Singapore Med J. 2009;50(6):614–8. [PubMed] [Google Scholar]
- 8.Reid VA, Adbulhadi H, Black KR, Kerrigan CDC. Using posturography to detect unsteadiness in 13 patients with peripheral neuropathy: a pilot study. Neurol Clin Neurophysiol. 2002;2002(4):2–8. doi: 10.1162/153840902760213658. [DOI] [PubMed] [Google Scholar]
- 9.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance. Phys Ther. 1986;66(10):1548–50. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 10.Fregly AR. Vestibular ataxia and its measurement in man. In: Kornhuber HH, editor. Handbook of Sensory Physiology. Berlin: Springer Verlag; 1974. pp. 321–60. [Google Scholar]
- 11.Fregly AR, Graybiel A, Smith MJ. Walk on Floor Eyes Closed (WOFEC): a new addition to an ataxia test battery. Aerosp Med. 1972;43(4):395–9. [PubMed] [Google Scholar]
- 12.Mayo Clinic DoN. Mayo Clinic Examinations in Neurology. 7. Rochester: Mayo Clinic Foundation; 1998. [Google Scholar]
- 13.Longridge NS, Mallinson AI. Clinical Romberg testing does not detect vestibular disease. Otol Neurotol. 2010;31(5):803–10. doi: 10.1097/MAO.0b013e3181e3deb2. [DOI] [PubMed] [Google Scholar]
- 14.Cohen HS, Mulavara AP, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ. Tests of walking balance for screening vestibular disorders. J Vestib Res. 2012;22:95–104. doi: 10.3233/VES-2012-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allum JHJ, Adkin AL, Carpenter MG, Held-Ziolkow M, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of a unilateral vestibular deficit. Gait Posture. 2001;14(3):227–37. doi: 10.1016/s0966-6362(01)00132-1. [DOI] [PubMed] [Google Scholar]
- 16.Gill J, Allum JHJ, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, et al. Trunk sway measures of postural stability during clinical balance test: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56A(7):M438–M47. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 17.Speers RA, Ashton-Miller JA, Schultz AB, Alexander NB. Age differences in abilities to perform tandem stand and walk tasks of graded difficulty. Gait Posture. 1998;7(3):207–13. doi: 10.1016/s0966-6362(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 18.Vereeck L, Wuyts F, Truijen S, Van de Heyning PH. Clinical assessment of balance: normative data, and gender and age effects. Int J Audiol. 2008;47(2):67–75. doi: 10.1080/14992020701689688. [DOI] [PubMed] [Google Scholar]
- 19.Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 20.Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM. 2006;8:19–20. doi: 10.1017/s1481803500013336. [DOI] [PubMed] [Google Scholar]
- 21.Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper R, Kuh D, Hardy R, Group MR Teams FaHS. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:4467. doi: 10.1136/bmj.c4467. Epub Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


