Abstract
AIM: To investigate the weight loss and glycemic control status [blood glucose, hemoglobin A1c (HbA1c) and hypoglycaemic treatment].
METHODS: The primary risk factor for type 2 diabetes is obesity, and 90% of all patients with type 2 diabetes are overweight or obese. Although a remarkable effect of bariatric surgery is the profound and durable resolution of type 2 diabetes clinical manifestations, little is known about the difference among various weight loss surgical procedures on diabetes remission. Data from patients referred during a 3-year period (from January 2009 to December 2011) to the University of Naples “Federico II” diagnosed with obesity and diabetes were retrieved from a prospective database. The patients were split into two groups according to the surgical intervention performed [sleeve gastrectomy (SG) and mini-gastric bypass (MGB)]. Weight loss and glycemic control status (blood glucose, HbA1c and hypoglycaemic treatment) were evaluated.
RESULTS: A total of 53 subjects who underwent sleeve gastrectomy or mini-gastric bypass for obesity and diabetes were screened for the inclusion in this study. Of these, 4 subjects were excluded because of surgical complications, 7 subjects were omitted because young surgeons conducted the operations and 11 subjects were removed because of the lack of follow-up. Thirty-one obese patients were recruited for this study. A total of 15 subjects underwent SG (48.4%), and 16 underwent MGB (51.6%). After adjusting for various clinical and demographic characteristics in a multivariate logistic regression analysis, high hemoglobin A1c was determined to be a negative predictor of diabetes remission at 12 mo (OR = 0.366, 95%CI: 0.152-0.884). Using the same regression model, MGB showed a clear trend toward higher diabetes remission rates relative to SG (OR = 3.780, 95%CI: 0.961-14.872).
CONCLUSION: Although our results are encouraging regarding the effectiveness of mini-gastric bypass on diabetes remission, further studies are needed to provide definitive conclusions in selecting the ideal procedure for diabetes remission.
Keywords: Bariatric surgery, Sleeve, Bypass, Obesity and diabetes
Core tip: Duodenum exclusion could suggest the potential superiority of mini-gastric bypass over sleeve gastrectomy to obtain diabetes remission. This mechanism could suggest the potential superiority of mini-gastric bypass over sleeve gastrectomy to obtain diabetes remission. Thus, although the gold standard for diabetes remission is still the Roux-en-y gastric by pass, being similar mechanisms of diabetes remission involved and being easier to be performed, the mini-gastric by pass could become a valuable alternative.
INTRODUCTION
Severe obesity is one of the major problems in Western Countries and is associated with several comorbidities and disabling diseases (e.g., cardiovascular disease, metabolic syndrome, type 2 diabetes, fertility, certain tumor types and increased mortality)[1-6].
One of the major comorbidities of obesity is type 2 diabetes mellitus (T2DM). In fact, the term “diabesity”[7] has been introduced to refer to obesity accompanied by T2DM.
With the exception of nutritional and some pharmacological treatments, bariatric surgery is performed more and more frequently as the treatment of choice in patients with severe obesity.
The efficacy of these surgical procedures in weight control has been widely described in several studies. Additionally, one of the most relevant corollary effects reported following bariatric surgery is T2DM remission.
A variety of surgical procedures are available and, currently, it is difficult to identify the most effective option based on patient characteristics and comorbidities. Furthermore, little is known regarding the effect of the various surgical procedures on glycemic control and on T2DM remission[8-11] .
The aim of this study is to compare the clinical efficacy of laparoscopic sleeve gastrectomy (SG) and laparoscopic mini-gastric bypass (MGB) in terms of T2DM remission.
MATERIALS AND METHODS
Data from patients referred during a 3-year period (from January 2009 to December 2011) to the University of Naples “Federico II” diagnosed with obesity and diabetes were retrieved from a prospective database.
Only patients who underwent uneventful laparoscopic SG or MGB with a follow-up of at least 1 year were included.
The patients were split into two groups according to the surgical intervention performed (SG and MGB).
Medical records were reviewed to collect the demographic and clinical characteristics of the patients. According to standard procedures[12], the study population was stratified based on abdominal obesity [body mass index (BMI) > 30 kg/m2], triglycerides levels (equal to or > 150 mg/dL), HDL-cholesterol (< 40 mg/dL for men and 50 mg/dL for women with total cholesterol values > 200 mg/dL), blood pressure (systolic blood pressure equal to or > 130 and/or diastolic > 85 mmHg) and fasting glucose levels (equal to or > 110 mg/dL).
Diagnosis of T2DM was made according to the American Diabetes Association guidelines. T2DM remission was defined as a fasting plasma glucose level below 126 mg/dL in the absence of hypoglycemic drugs.
The indications for treating these patients were the same as those published in the Italian Society for Bariatric Surgery guidelines[13]. Following the failure of a non-operative weight reduction program that included diet and other interventions (e.g., behavioral modification, psychotherapy, dietary counseling, or physical training), bariatric surgery plays a critical role in patient outcome when the BMI is > 40 kg/m2 or is > 35 kg/m2 combined with serious co-existing conditions.
Only surgical procedures performed by an expert surgeon (more than 500 laparoscopic surgical procedures) were included in the analysis.
Prevention of surgical site infection and perioperative antiplatelet drug administration were managed according to validated criteria[14,15].
For the sleeve gastrectomy procedure, 75%-80% of the greater curvature was excised, leaving a narrow stomach tube of 38F. Single-loop gastric bypass was performed, which consisted of constructing a 40-70 mL sleeve gastric pouch with a jejunal exclusion of 200-220 cm. All procedures were performed using a laparoscopic approach[16-18].
Following surgery, clinical controls were performed once a month for the first 3 postoperative months and every 3 mo thereafter.
At each follow-up visit, weight loss and glycemic control status [blood glucose, hemoglobin A1c (HbA1c) and hypoglycemic treatment] were evaluated. Diabetes remission was defined as HbA1c values less than 6.5 without the use of oral hypoglycemic treatment or insulin[11,19].
Statistical analysis was performed using the SPSS 17 system (SPSS Inc., Chicago, IL, United States). Continuous data were expressed as the mean ± SD, and categorical variables were expressed as the percent changes. To compare continuous variables, an independent and/or paired sample t test was performed, and correlation was assessed using the Pearson’s linear correlation coefficients (r). Changes in BMI, glycemia and HbA1c were expressed as the percent changes vs baseline values. The χ2 test was used to analyze categorical data. When the minimum expected value was < 5, the Fisher’s exact test was used. To adjust for major covariates and to generate predictions, a logistic regression (stepwise) model was applied, with diabetes remission at 12 mo as the dependent variable and gender, age, hypertension, hypercholesterolemia, current hypoglycemia treatment, BMI baseline value, glycemia and HbA1c as independent variables. All of the results are presented as 2-tailed values with statistical significance defined as P values < 0.05.
RESULTS
A total of 53 subjects who underwent sleeve gastrectomy or mini-gastric bypass for obesity and diabetes were screened for the inclusion in this study. Of these, 4 subjects were excluded because of surgical complications, 7 subjects were omitted because young surgeons conducted the operations and 11 subjects were removed because of the lack of follow-up. Thus, a total of 31 obese patients (15 males and 16 females; mean age: 38.32 ± 3.21 years; BMI: 44.78 ± 4.25 kg/m2) were recruited for this study. All patients were diagnosed with type 2 diabetes [15 (48.4%) on metformin and 16 (51.6%) on metformin + insulin], 18 subjects (58.1%) reported hypertension and 8 presented with hypercholesterolemia. The mean glycemia value was 169.87 ± 35.76, and the mean HbA1c level was 8.5 ± 1.0. A total of 15 subjects underwent SG (48.4%), and 16 patients underwent MGB (51.6%). Major clinical and demographic characteristics of the study population stratified according to type of surgery are reported in Table 1.
Table 1.
Baseline clinical and demographic characteristics of the study population n (%)
| Sleeve (n = 15) | Mini-bypass (n = 16) | P value | |
| Age | 37.26 ± 3.7 | 39.3 ± 2.3 | 0.076 |
| Male gender | 7 (46.7) | 8 (50.0) | 1.000 |
| BMI | 43.6 ± 2.99 | 45.8 ± 5.0 | 0.140 |
| Diabetes treatment | |||
| Metformin ± insulin | 7 (46.7) | 9 (56.3) | 0.724 |
| Metformin | 8 (53.3) | 7 (43.7) | |
| Glycemia | 161.4 ± 31.4 | 177.8 ± 38.6 | 0.207 |
| HbA1c | 8.6 ± 1.0 | 8.47 ± 1.1 | 0.782 |
| Hypertension | 8 (53.3) | 10 (62.5) | 0.722 |
| Diabetes | 15 (100.0) | 16 (100.0) | 1.000 |
| Hypercholesterolemia | 4 (26.6) | 4 (25.0) | 1.000 |
BMI: Body mass index; HbA1c: Hemoglobin A1c.
After surgical intervention, a significant and consistent reduction in BMI, glycemia and HbA1c values were observed relative to the baseline values (Figure 1). Stratifying for type of surgery, SG and MGB were associated with similar percent changes in BMI (-24.33 ± 4.48 vs -24.19 ± 4.42, P = 0.931), glycemia (-24.30 ± 11.40 vs -28.42 ± 14.03, P = 0.379) and HbA1c (-22.57 ± 8.70 vs -22.67 ± 8.46, P = 0.975).
Figure 1.
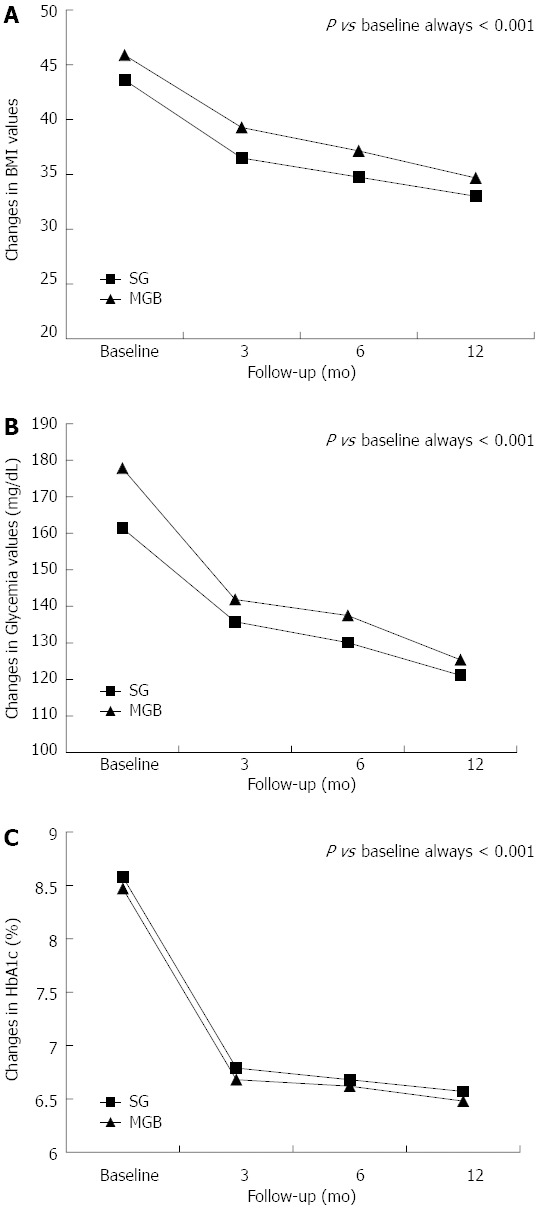
Changes in body mass index (A), glycemia (B) and hemoglobin A1c (C) values following surgery. HbA1c: Hemoglobin A1c; SG: Sleeve gastrectomy; MGB: Mini-gastric bypass; BMI: Body mass index.
Overall, significant correlations were not detected in the percent change from baseline to 12-mo follow-up between BMI and glycemia, as well as between BMI and HbA1c (Figure 2). Additionally, the same results were confirmed after stratifying based on the type of surgery. Indeed, the percent change in BMI did not correlate with changes in glycemia (r = -0.119, P = 0.673 for SG and r = 0.462, P = 0.071 for MGB) or with changes in HbA1c (r = -0.349, P = 0.202 for SG and r = -0.018, P = 0.946 for MGB).
Figure 2.
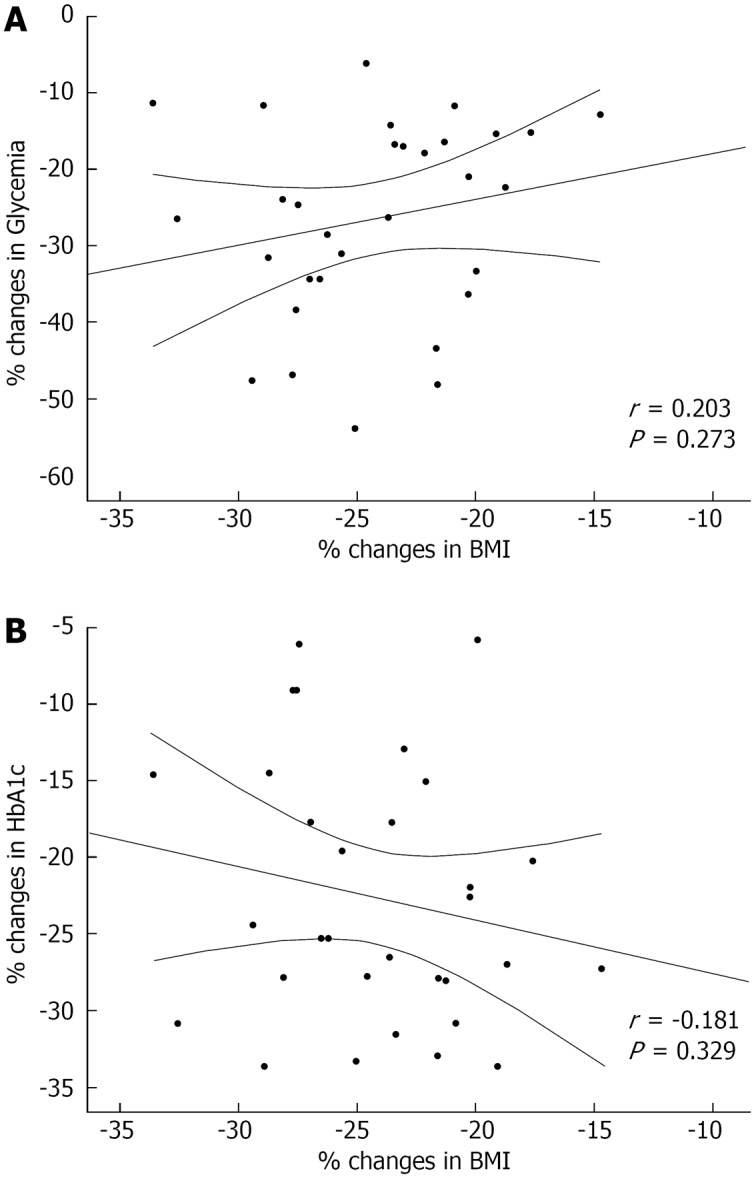
Scatter plot of Pearson’s correlations between the percent change in glycemia and body mass index (A) and in hemoglobin A1c and body mass index (B) following surgical intervention. HbA1c: Hemoglobin A1c; BMI: Body mass index.
As shown in Figure 3, the prevalence of diabetes remission was gradually increased following surgery, regardless of the type.
Figure 3.
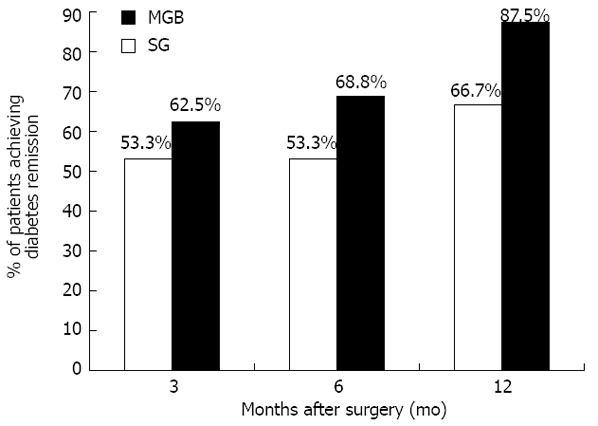
Prevalence of subjects achieving diabetes remission in the sleeve gastrectomy group and the mini-gastric bypass group. SG: Sleeve gastrectomy; MGB: Mini-gastric bypass.
Specifically, at 3 mo post-surgical intervention, diabetes remission was reported by 18 subjects (53.3% in SG vs 62.5% in MGB, P = 0.722). Similar results were confirmed at the 6-mo follow-up (53.3% for SG vs 68.8% for MGB, P = 0.473)
At the 12-mo follow-up, 66.7% of subjects who underwent SG achieved diabetes remission vs 87.5% of those who underwent MGB (P = 0.220).
Interestingly, the percent change in BMI was similar between patients achieving diabetes remission and patients who did not (-24.28 ± 4.33 vs -24.15 ± 4.53, respectively, P = 0.97).
After adjusting for various clinical and demographic characteristics in a multivariate logistic regression analysis, a high HbA1c was considered a negative predictor of diabetes remission at 12 mo (OR = 0.366, 95%CI: 0.152-0.884). Using the same regression model, MGB showed a clear trend towards a higher diabetes remission rate relative to SG (OR = 3.780, 95%CI: 0.961-14.872).
DISCUSSION
The prevalence of type 2 diabetes has markedly increased in the last decade, both in the United States[20-23] and globally[24-26]. These data are correlated with a comparably steep increase in the prevalence of obesity[27-29]. The primary risk factor for type 2 diabetes is obesity, and 90% of all patients with type 2 diabetes are either overweight or obese[30,31]. The National Health and Nutrition Examination Survey III (1988-1994) data demonstrated that the risk for chemical diabetes is approximately 50% with a BMI of greater than or equal to 30 kg/m2 and over 90% with a BMI of 40 kg/m2 or more[32]. The Nurses’ Health Study that was conducted on 84941 women (1980-1996) showed that the relative risk of diabetes increased approximately 40-fold as the BMI increased from less than 23 kg/m2 to more than 35 kg/m2[33].
Morbid obesity has been defined by the National Institutes of Health as a BMI of greater than or equal to 40 kg/m2 or greater than or equal to 35 kg/m2 in the presence of obesity comorbidities[13,19,20].
The prevalence of people who are overweight or obese has increased dramatically in high-income countries during the past 20 years. The World Health Organization estimates that 54.3% of women and 51.7% of men in the United States will be obese (BMI 30 kg/m2) in 2015.
Obesity is notoriously difficult to manage. Diet, behavioral therapy, exercise, and pharmacologic intervention have traditionally been used but generally yield modest results. Additionally, weight regain is a common problem. In cases of failed medical therapy, bariatric surgery should be considered the treatment of choice for severe obesity[34].
Surgical treatment of obesity is a rapidly growing area of surgical practice, reflecting the ability of bariatric surgery to achieve significant and durable weight loss, as well as the evolution of safer, less-invasive procedures[35-37].
A remarkable effect of bariatric surgery is the profound and durable resolution of type 2 diabetes clinical manifestations. In a meta-analysis of 134 studies that reported comorbidity resolution (2738 citations), bariatric surgery followed by resolution of type 2 diabetes was observed in 48% of patients who underwent laparoscopic adjustable gastric banding, 84% of patients who underwent gastric bypass, and 98% of patients who underwent biliopancreatic diversion/duodenal switch[38]. These data were based on reports from 22094 patients from January 1, 1990 to June 5, 2003.
Roux-en-Y gastric bypass (RYGB) is considered the gold standard bariatric procedure for achieving diabetes remission.
To explain the mechanisms underlying the effectiveness of gastric bypass procedures in normalizing glycemia, it has been suggested that removal of the gut may play a major role in diabetes remission, especially because important hormones are secreted from this region. In 2009, Cummings reviewed the existing hypotheses regarding the mechanisms underlying diabetes remission. Based on this review, the main hypotheses include the ghrelin hypothesis, the upper intestinal hypothesis and the lower intestinal hypothesis. The ghrelin hypothesis[39] maintains that gherlin regulation may be disturbed following RYGB. Ghrelin is a hormone secreted by the stomach and proximal small bowel, particularly before meals. Its main physiological effects include increased appetite and fat mass increase[40]. In support of the ghrelin hypothesis, several studies have shown that ghrelin levels are very low following RYGB. Diminished ghrelin secretion can decrease appetite and food intake, and reduced secretion might also have a role in increasing glucose tolerance, as ghrelin can stimulate counter-regulatory hormones[41]. The lower intestinal hypothesis claims that intestinal shortcuts, created by bariatric surgery, expedite delivery of ingested nutrients and increase glucagon-like peptide-1 (GLP-1) release. GLP-1 is an incretin, defined as a peptide secreted from enteroendocrine L-cells. These cells are found throughout the small intestine and at a high density in the ileum. GLP-1 increases insulin secretion and has also been shown to increase proliferation and decrease apoptosis of beta-cells[42]. Both RYGB and BPD create gastrointestinal shortcuts, and studies have shown that postprandial GLP-1 secretion is increased post-surgery[43,44]. Therefore, it seems reasonable that, following surgery, GLP-1 secretion may be enhanced, leading to enhanced insulin secretion. This mechanism could also explain the increase in β-cell mass that is thought to accompany post-RYGB hyperinsulinemic hypoglycemia[45]. The upper intestinal hypothesis maintains that avoiding nutrient contacts with the duodenum is somehow key in the process through which diabetes is improved. The basis of this hypothesis is that unknown factors or processes from the duodenum influence glucose homeostasis[39]. Rubino and Marescaux[46] were the first to provide support for this hypothesis. They experimented on a variant of RYGB creating the intestinal bypass but leaving the stomach intact, which induced the same digestive discontinuation without reanastomosis. This surgery, termed duodenal-jejunal bypass, was tested in several studies showing an improvement in T2DM without reduction in body weight[41]. These studies suggest that the exclusion of the proximal intestine may play a role in diabetes remission.
Interestingly, sleeve gastrectomy and mini-gastric bypass have emerged as new and effective weight loss procedures[8-11,16-18,47,48].
There is increasing evidence indicating that SG causes early and significant improvements in glucose homeostasis in most morbidly obese subjects with T2DM[49-52].
A systematic review of the existing literature showed that SG results in T2DM resolution ranging from 80% to 96% in morbidly obese subjects[50], a range similar to that in patients following RYGBP[38].
Similarly, laparoscopic mini-gastric bypass is reported to be a safe alternative to LRYGB, showing comparable efficacy in weight reduction and resolution of metabolic complications, including diabetes[52-54].
Both short-term[55-57] and long-term[58,59] follow-up confirmed the durable effect of this simplified procedure for obese or morbidly obese patients with T2DM.
Laparoscopic mini-gastric bypass in morbidly obese patients with T2DM has been shown to be effective in prospective randomized controlled trials[55], as well as in extensive reports in the literature[54-59].
Gherlin regulation is disturbed following the sleeve gastrectomy procedure. SG was also reported to have a hindgut effect with increasing levels of glucagon-like peptide 1 and peptide YY due to the increased transit time after SG[60].
Because construction of the sleeve gastric pouch is the first step of this technique, similar mechanisms are involved in mini-gastric bypass procedure.
The only region unaffected by SG was on the foregut. Specifically, the upper intestine was not in contact with ingested nutrients in the GB-treated group, while contact was made in the SG-treated group.
Recently, Lee et al[9] published the first comparative study between sleeve gastrectomy and mini-gastric bypass to determine the efficacy of these treatments on diabetic control. Their results strongly support the hypothesis that duodenal exclusion may play a role in diabetes mellitus resolution following bariatric surgery in overweight patients.
Our findings extend the observations of Lee to severely obese patients. Unlike the study conducted by Lee et al[9], we only enrolled patients diagnosed with severe obesity and a clear indication to bariatric surgery. Despite this difference in the recruited patient population, our results also confirm that MGB is associated with better glycemic control and a higher rate of diabetes remission.
Although we observed a clear trend in our study, this did not achieve statistical significance. A multivariate analysis was performed to adjust for major clinical and demographic variables, but because of the relatively small sample size, our results need to be validated in larger studies. Thus, the present work could be considered a preliminary study, providing the rationale for a randomized prospective trial.
Further supporting this hypothesis, we reported that BMI changes were similar between patients achieving diabetes remission and patients not attaining remission. This finding suggests that diabetes remission may be independent from weight loss and that the type of surgery may play a more relevant role. These results combined with the evidence that weight loss is similar following the sleeve gastrectomy and mini-gastric bypass procedures may still support the theory suggested by Lee et al[9]. Consequently, if we exclude the role of weight loss and both the ghrelin and hindgut theories, the only remaining theory to explain the different results is the foregut (duodenal exclusion).
Overall, this mechanism suggests a potential superiority of the mini-gastric bypass over the sleeve gastrectomy in obtaining diabetes remission, but further data are needed to make this conclusion.
While the gold standard for diabetes remission remains the Roux-en-y gastric bypass because similar mechanisms of diabetes remission may be involved and the procedure is easier to perform, the mini-gastric bypass could become a valuable alternative.
Although our results appear to be encouraging and support mini-gastric bypass as an effective treatment strategy for diabetes remission, further studies are needed to allow for definitive conclusions regarding the ideal procedure for obtaining diabetes remission.
COMMENTS
Background
A remarkable effect of bariatric surgery is the profound and durable resolution of type 2 diabetes clinical manifestations. Interestingly, although both sleeve gastrectomy and mini-gastric bypass have emerged as new and effective weight loss procedures, the duodenal exclusion (involved in the mini-gastric bypass) may play a role in diabetes mellitus resolution.
Research frontiers
The encouraging results obtained in this study with the mini-gastric bypass provide the rationale for a future randomized prospective trial to validate the effectiveness of this surgical technique.
Innovations and breakthroughs
Their results confirm that mini-gastric bypass is associated with better glycemic control and higher diabetes remission rates relative to sleeve gastrectomy. The gold standard for diabetes remission remains Roux-en-y gastric bypass. However being similar mechanisms of diabetes remission involved and being easier to be performed, the mini-gastric by pass could become a valuable alternative.
Applications
Mini-gastric bypass should be considered an effective weight loss procedure in diabetic patients.
Peer review
The results are interesting and suggest that the effectiveness of mini-gastric bypass on diabetes remission, further studies are needed to provide definitive conclusions in selecting the ideal procedure for diabetes remission.
Footnotes
P- Reviewers Koch TR, Wig JD S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Toghaw P, Matone A, Lenbury Y, De Gaetano A. Bariatric surgery and T2DM improvement mechanisms: a mathematical model. Theor Biol Med Model. 2012;9:16. doi: 10.1186/1742-4682-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 4.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 5.Musella M, Milone M, Bellini M, Fernandez ME, Fernandez LM, Leongito M, Milone F. The potential role of intragastric balloon in the treatment of obese-related infertility: personal experience. Obes Surg. 2011;21:426–430. doi: 10.1007/s11695-010-0167-2. [DOI] [PubMed] [Google Scholar]
- 6.Musella M, Milone M, Bellini M, Sosa Fernandez LM, Leongito M, Milone F. Effect of bariatric surgery on obesity-related infertility. Surg Obes Relat Dis. 2012;8:445–449. doi: 10.1016/j.soard.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg. 2009;19:217–229. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143–148. doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- 9.Lee WJ, Wang W, Lee YC, Huang MT, Ser KH, Chen JC. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI > 35 and < 35 kg/m2. J Gastrointest Surg. 2008;12:945–952. doi: 10.1007/s11605-007-0319-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim Z, Hur KY. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J Surg. 2011;35:631–636. doi: 10.1007/s00268-010-0909-2. [DOI] [PubMed] [Google Scholar]
- 11.Cutolo PP, Nosso G, Vitolo G, Brancato V, Capaldo B, Angrisani L. Clinical efficacy of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. Obes Surg. 2012;22:1535–1539. doi: 10.1007/s11695-012-0657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Minno MN, Tufano A, Guida A, Di Capua M, De Gregorio AM, Cerbone AM, Tarantino G, Di Minno G. Abnormally high prevalence of major components of the metabolic syndrome in subjects with early-onset idiopathic venous thromboembolism. Thromb Res. 2011;127:193–197. doi: 10.1016/j.thromres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 13. Available from: http://www.sicob.org.
- 14.Di Minno MN, Milone M, Mastronardi P, Ambrosino P, Di Minno A, Parolari A, Tremoli E, Prisco D. Perioperative handling of antiplatelet drugs. A critical appraisal. Curr Drug Targets. 2013;14:880–888. doi: 10.2174/1389450111314080008. [DOI] [PubMed] [Google Scholar]
- 15.Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10:317–328. doi: 10.1586/erp.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, Ramanathan R, Schauer P. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–863. doi: 10.1007/s00464-005-0134-5. [DOI] [PubMed] [Google Scholar]
- 17.Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11:276–280. doi: 10.1381/096089201321336584. [DOI] [PubMed] [Google Scholar]
- 18.Milone M, Di Minno MN, Galloro G, Maietta P, Bianco P, Milone F, Musella M. Safety and efficacy of barbed suture for gastrointestinal suture: a prospective and randomized study on obese patients undergoing gastric bypass. J Laparoendosc Adv Surg Tech A. 2013;23:756–759. doi: 10.1089/lap.2013.0030. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Levi AM, Cabrerizo L, Matía P, Sánchez-Pernaute A, Torres AJ, Rubio MA. Which criteria should be used to define type 2 diabetes remission after bariatric surgery? BMC Surg. 2013;13:8. doi: 10.1186/1471-2482-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 22.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 23.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 24.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 25.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 26.The World Health Report 2006. Working Together for Health. Geneva, Switzerland: Geneva World Health Organization; 2006. [Google Scholar]
- 27.World Watch Institute. Chronic hunger and obesity epidemics eroding global progress. Available from: http://www.worldwatch.org/node/1672. Accessed January 2, 2007.
- 28.World Health Organization. The global challenge of obesity, 2002. Available from: http://www.who.int/dietphysicalactivity/en/ Accessed January 2, 2007.
- 29.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 30.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 31.North American Association for the Study of Obesity (NAASO) and the National Heart, Lung, and Blood Institute (NHLBI) The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH Publication 00-4084, Oct 2000. Available from: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf.
- 32.NHANES III Public Use Data File. U.S. Department of Health and Human Service (DHHS). National Center for Health Statistics. Third National Health and Nutrition Examination Survey, 1988-1994, NHANES III Laboratory Data File (CD-ROM). Public Use Data File Documentation Number 76200. Hyattsville (MD): Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 33.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 34.2004 ASBS Consensus Conference on Surgery for Severe Obesity. Surg Obes Relat Dis. 2005;1:297–381. doi: 10.1016/j.soard.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien PE, Dixon JB, Brown W. Obesity is a surgical disease: overview of obesity and bariatric surgery. ANZ J Surg. 2004;74:200–204. doi: 10.1111/j.1445-2197.2004.03014.x. [DOI] [PubMed] [Google Scholar]
- 36.Wax JR, Pinette MG, Cartin A, Blackstone J. Female reproductive issues following bariatric surgery. Obstet Gynecol Surv. 2007;62:595–604. doi: 10.1097/01.ogx.0000279291.86611.46. [DOI] [PubMed] [Google Scholar]
- 37.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 38.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 39.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33 Suppl 1:S33–S40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 40.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 41.Cummings DE, Overduin J, Foster-Schubert KE, Carlson MJ. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis. 2007;3:109–115. doi: 10.1016/j.soard.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 46.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scarano V, Milone M, Di Minno MN, Panariello G, Bertogliatti S, Terracciano M, Orlando V, Florio C, Leongito M, Lupoli R, et al. Late micronutrient deficiency and neurological dysfunction after laparoscopic sleeve gastrectomy: a case report. Eur J Clin Nutr. 2012;66:645–647. doi: 10.1038/ejcn.2012.10. [DOI] [PubMed] [Google Scholar]
- 48.Piazza L, Ferrara F, Leanza S, Coco D, Sarvà S, Bellia A, Di Stefano C, Basile F, Biondi A. Laparoscopic mini-gastric bypass: short-term single-institute experience. Updates Surg. 2011;63:239–242. doi: 10.1007/s13304-011-0119-y. [DOI] [PubMed] [Google Scholar]
- 49.Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26:2231–2239. doi: 10.1007/s00464-012-2166-y. [DOI] [PubMed] [Google Scholar]
- 50.Gill RS, Karmali S, Sharma AM. Treating type 2 diabetes mellitus with sleeve gastrectomy in obese patients. Obesity (Silver Spring) 2011;19:701–702. doi: 10.1038/oby.2010.261. [DOI] [PubMed] [Google Scholar]
- 51.Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24:1005–1010. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 52.Reis CE, Alvarez-Leite JI, Bressan J, Alfenas RC. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index < 35 kg/m2: a literature review. Diabetes Technol Ther. 2012;14:365–372. doi: 10.1089/dia.2011.0127. [DOI] [PubMed] [Google Scholar]
- 53.Chakhtoura G, Zinzindohoué F, Ghanem Y, Ruseykin I, Dutranoy JC, Chevallier JM. Primary results of laparoscopic mini-gastric bypass in a French obesity-surgery specialized university hospital. Obes Surg. 2008;18:1130–1133. doi: 10.1007/s11695-008-9594-8. [DOI] [PubMed] [Google Scholar]
- 54.Lee WJ, Huang MT, Wang W, Lin CM, Chen TC, Lai IR. Effects of obesity surgery on the metabolic syndrome. Arch Surg. 2004;139:1088–1092. doi: 10.1001/archsurg.139.10.1088. [DOI] [PubMed] [Google Scholar]
- 55.Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005;242:20–28. doi: 10.1097/01.sla.0000167762.46568.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Wei PL, Lee YC, Huang MT, Chiu CC, Lee WJ. Short-term results of laparoscopic mini-gastric bypass. Obes Surg. 2005;15:648–654. doi: 10.1381/0960892053923752. [DOI] [PubMed] [Google Scholar]
- 57.Lee WJ, Ser KH, Lee YC, Tsou JJ, Chen SC, Chen JC. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012;22:1827–1834. doi: 10.1007/s11695-012-0726-9. [DOI] [PubMed] [Google Scholar]
- 58.Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15:1304–1308. doi: 10.1381/096089205774512663. [DOI] [PubMed] [Google Scholar]
- 59.Lee WJ, Lee YC, Ser KH, Chen SC, Chen JC, Su YH. Revisional surgery for laparoscopic minigastric bypass. Surg Obes Relat Dis. 2011;7:486–491. doi: 10.1016/j.soard.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–418. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]


