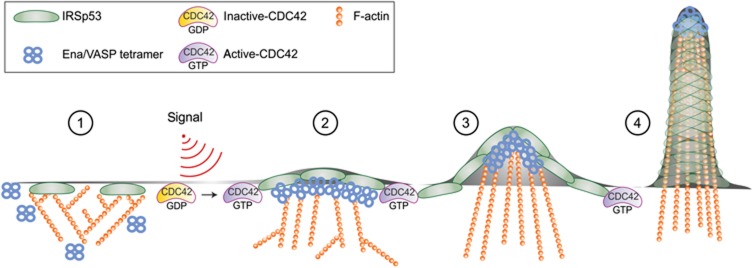
Figure 8.
Model depicting how CDC42 switches IRSp53 from inhibition of actin assembly to filament elongation by clustering VASP during filopodia formation. A series of distinct signalling and biochemically defined steps characterize the initial phases of filopodia formation, mediated by the membrane deforming protein IRSp53 and its interactor VASP. Step 1: in the absence of stimuli, IRSp53 binds to the PM and slows down barbed end elongation. The constitutive association of IRSp53 with the capping protein EPS8 likely reinforces EPS8 capping activity (not shown) (Disanza et al, 2006; Vaggi et al, 2011). Step 2: following filopodia-inducing stimuli, activated CDC42 binds to IRSp53. This interaction relieves IRSp53-mediated inhibition of filament growth and promotes the formation of a CDC42:IRSp53:VASP complex, and simultaneously reduces the formation of the IRSP53:EPS8 complex. Step 3: binding to CDC42 further facilitates the formation of IRSp53 and VASP foci at the leading edge of the PM, in which VASP clustering allows processive actin filament elongation in the presence of capping protein. Step 4: membrane deforming activity of IRSp53 and processive filament elongation by VASP work in concert to extend actin filaments beyond the x, y plane of the membrane. During this process, the filaments become cross-linked by fascin (not shown), to resist counter membrane tension and extracellular compression forces.