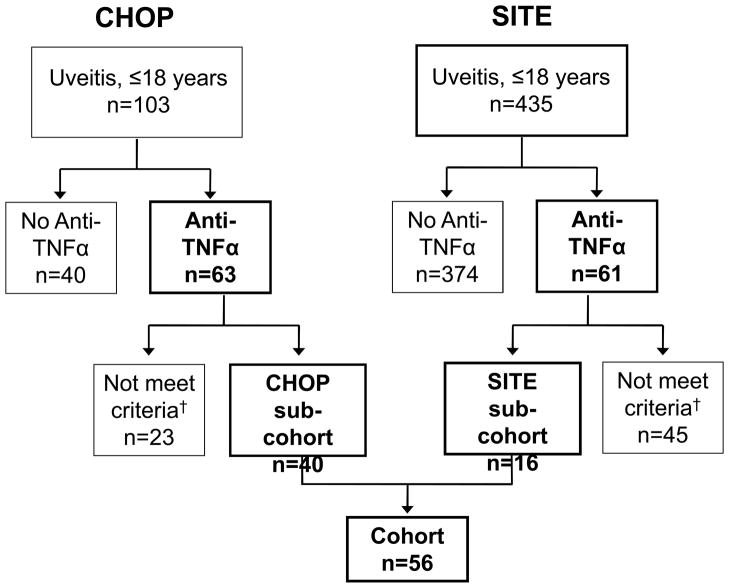
Figure 1.
Study cohort. Of 538 patients treated for non-infectious uveitis, 124 were treated with anti-TNFα. 56 of these met inclusion criteria for anti-TNFα/Corticosteroid/Immunomodulatory therapy use. †68 were excluded (23 potential subjects from CHOP and 45 from SITE) due to: inadequate records (did not have a visit within 30 days prior to initiation of anti-TNFα; no documentation of uveitis status at onset of anti-TNFα therapy; uveitis that developed after starting anti-TNFα; or initiation of anti-TNFα after the cut-off for the study). SITE=Systemic Immunosuppressive Therapy for Eye Disease Cohort Study; CHOP=The Children’s Hospital of Philadelphia; anti-TNFα, Tumor Necrosis Factor-α inhibitor.