Abstract
Mild traumatic brain injury (mTBI), also referred to as concussion, remains a controversial diagnosis because the brain often appears quite normal on conventional computed tomography (CT) and magnetic resonance imaging (MRI) scans. Such conventional tools, however, do not adequately depict brain injury in mTBI because they are not sensitive to detecting diffuse axonal injuries (DAI), also described as traumatic axonal injuries (TAI), the major brain injuries in mTBI. Furthermore, for the 15 to 30% of those diagnosed with mTBI on the basis of cognitive and clinical symptoms, i.e., the “miserable minority,” the cognitive and physical symptoms do not resolve following the first three months post-injury. Instead, they persist, and in some cases lead to long-term disability. The explanation given for these chronic symptoms, i.e., postconcussive syndrome, particularly in cases where there is no discernible radiological evidence for brain injury, has led some to posit a psychogenic origin. Such attributions are made all the easier since both post-traumatic stress disorder (PTSD) and depression are frequently co-morbid with mTBI. The challenge is thus to use neuroimaging tools that are sensitive to DAI/TAI, such as diffusion tensor imaging (DTI), in order to detect brain injuries in mTBI. Of note here, recent advances in neuroimaging techniques, such as DTI, make it possible to characterize better extant brain abnormalities in mTBI. These advances may lead to the development of biomarkers of injury, as well as to staging of reorganization and reversal of white matter changes following injury, and to the ability to track and to characterize changes in brain injury over time. Such tools will likely be used in future research to evaluate treatment efficacy, given their enhanced sensitivity to alterations in the brain. In this article we review the incidence of mTBI and the importance of characterizing this patient population using objective radiological measures. Evidence is presented for detecting brain abnormalities in mTBI based on studies that use advanced neuroimaging techniques. Taken together, these findings suggest that more sensitive neuroimaging tools improve the detection of brain abnormalities (i.e., diagnosis) in mTBI. These tools will likely also provide important information relevant to outcome (prognosis), as well as play an important role in longitudinal studies that are needed to understand the dynamic nature of brain injury in mTBI. Additionally, summary tables of MRI and DTI findings are included. We believe that the enhanced sensitivity of newer and more advanced neuroimaging techniques for identifying areas of brain damage in mTBI will be important for documenting the biological basis of postconcussive symptoms, which are likely associated with subtle brain alterations, alterations that have heretofore gone undetected due to the lack of sensitivity of earlier neuroimaging techniques. Nonetheless, it is noteworthy to point out that detecting brain abnormalities in mTBI does not mean that other disorders of a more psychogenic origin are not co-morbid with mTBI and equally important to treat. They arguably are. The controversy of psychogenic versus physiogenic, however, is not productive because the psychogenic view does not carefully consider the limitations of conventional neuroimaging techniques in detecting subtle brain injuries in mTBI, and the physiogenic view does not carefully consider the fact that PTSD and depression, and other co-morbid conditions, may be present in those suffering from mTBI. Finally, we end with a discussion of future directions in research that will lead to the improved care of patients diagnosed with mTBI.
Keywords: Mild Traumatic Brain Injury, mTBI, TBI, Diffusion Tensor Imaging, DTI, Magnetic Resonance Imaging, MRI, Diffusion-Weighted Imaging, DWI, Susceptibility-Weighted Imaging, SWI, Signature Injury of War, Concussion, Postconcussive syndrome, Postconcussive Symptoms, Complicated mTBI, Uncomplicated mTBI, Physiogenesis, Psychogenesis, Miserable Minority
Introduction
The Scope of the Problem
More than 1.7 million people each year in the United States experience a traumatic brain injury (TBI), with 75 to 85% of these injuries categorized as mild (mTBI; CDC 2010; Faul et al., 2010; Bazarian et al., 2006). This number is likely an underestimate because it does not include those who are seen in private clinics or by primary care physicians, nor does it include those who do not seek medical treatment (Langlois et al., 2006). It is estimated, in fact, that 14% of mTBI patients are seen in private clinics or by their own doctors, with an additional 25% receiving no medical attention (Sosin et al, 1996). Based on the large number of known and likely unknown cases, traumatic brain injury has been referred to as the “silent epidemic” (e.g., Goldstein et al., 1990). Recently, the public has become more aware of TBI based on news reports of sports injuries leading to long-term effects of repetitive trauma to the brain, as well as news reports about soldiers returning from Iraq and Afghanistan with TBI. With respect to the latter, the most frequent combat-related injury incurred by soldiers returning from Iraq and Afghanistan is TBI, and most particularly mTBI (Okie et al., 2005). The frequency of these injuries has led to TBI being called the “signature injury of war” (Okie et al., 2005). Further, approximately 22% of the wounded soldiers arriving at Lundstuhl Regional Medical Center in Germany have head, neck, or face injuries, with cases of TBI resulting primarily from improvised explosive devices (IEDs), landmines, high pressure waves from blasts, blunt force injury to the head from objects in motion, and motor vehicle accidents (Okie et al., 2005; Warden et al., 2006). Of particular note, mTBI characterizes most of the blast-induced traumatic brain injuries seen in service members returning from Iraq and Afghanistan, with reports of 300,000 service members sustaining at least one mTBI as of 2008 (Tanielian and Jaycox, 2008). Mild TBI is thus a major health problem that affects both civilians and military populations. The estimated economic cost is also enormous, with mTBI accounting for 44% of the 56 billion dollars spent annually in the United States in treating TBI (Thurman, 2001).
Lack of Radiological Evidence
Mild TBI is, however, difficult to diagnose because often the brain appears quite normal on conventional computed tomography (CT) and magnetic resonance imaging (MRI) (e.g., Bazarian et al., 2007; Inglese et al., 2005; Hughes et al., 2004; Iverson et al., 2000; Miller et al., 1996; Mittl et al., 1994; Povlishock et al., 1989; Scheid et al., 2003). This lack of radiological evidence of brain injury in mTBI has led clinicians typically to diagnose mTBI on the basis of clinical and cognitive symptoms, which are generally based on self-report, and are non-specific as they overlap with other diagnoses (e.g., Hoge et al., 2008; Stein et al., 2009). To complicate matters further, while most of the symptoms in mTBI are transient and resolve within days to weeks, approximately 15 to 30% of patients evince cognitive, physiological, and clinical symptoms that do not resolve 3 months post-injury (e.g., Alexander 1995; Bazarian et al., 1999; Bigler 2008; Rimel et al., 1981; Vanderploeg et al., 2007). Instead, these symptoms persist and in some cases lead to permanent disability (Carroll et al., 2004a&b; Nolin et al., 2006), and to what has been referred to as persistent postconcussive symptoms (PPCS), or postconcussive syndrome (PCS), although the latter term, “PCS,” is controversial (e.g., Arciniegas et al., 2005).
This “miserable minority” (Ruff et al., 1996) often experience persistent postconcussive symptoms (PPCS) that include dizziness, headache, irritability, fatigue, sleep disturbances, nausea, blurred vision, hypersensitivity to light and noise, depression, anxiety, as well as deficits in attention, concentration, memory, executive function, and speed of processing (e.g., Bigler, 2008). Kurtzke (1993) estimates the incidence of persistent symptoms as being equal to the annual incidence of Parkinson’s disease, Multiple Sclerosis, Guillain-Barré Syndrome, Motor Neuron Disease, and Myasthenia Gravis, combined. Moreover, the modal age for injury is young, in the 20’s and 30’s. Thus mTBI affects a large number of individuals in the prime of life, where there is, to date, no consistent or reliable correlations between cognitive/clinical symptoms and radiological evidence of brain injury based on conventional neuroimaging.
The explanation given for PPCS, particularly when there is no discernible radiological evidence, has led some to posit a psychogenic origin (e.g., Belanger et al., 2009; Hoge et al., 2008; Lishman et al., 1988; Machulda et al., 1998). More specifically, Hoge and colleagues (2008, 2009) suggest that postconcussive symptoms reported by soldiers with mTBI are largely or entirely mediated by posttraumatic stress disorder (PTSD) and depression. In their study, after controlling for both PTSD and depression, the only remaining symptom was headaches. Headaches, nonetheless, are an important symptom of TBI, particularly mTBI.
The term “miserable minority,” described above, has been used to identify those who likely have a more psychogenic etiology to their symptoms (e.g., Ruff et al., 1996). Such attributions are easy to make given that the symptoms of mTBI, as noted above, overlap with other disorders (e.g., Hoge et al., 2008). Belanger et al. (2009) also suggest that most of the symptoms reported by those with mTBI are likely the result of emotional distress. Others have also argued that emotional distress and/or psychiatric problems account for those who continue to experience postconcussive symptoms (e.g., Belanger et al., 2009; Greiffenstein, 2008; Hoge et al., 2008; Lishman et al., 1988; Machulda et al., 1998).
Persistent symptoms, however, may be the result of more subtle neurological alterations that are beneath the threshold of what can be detected using conventional neuroimaging techniques that all too often do not reveal brain pathology in mTBI (e.g., Hayes et al., 1994; Huisman et al., 2004; Fitzgerald and Crosson, 2011; Green et al., 2010; Miller et al., 1996; Niogi et al., 2010). This is not at all surprising, since conventional techniques are not sensitive to detecting diffuse/traumatic axonal injuries (DAI/TAI), the major brain injuries observed in mTBI (e.g., Benson et al., 2007).
There is also evidence from the literature to suggest that in several cases of mTBI where there was no radiological evidence of brain injury, autopsy following death from injuries other than mTBI revealed microscopic diffuse axonal injuries that conventional neuroimaging tools did not detect, presumably because they were not sufficiently sensitive (e.g., Adams et al., 1989; Bigler et al., 2004; Blumbergs et al., 1994; Oppenheimer et al., 1968).
We would argue that the controversy between mTBI being psychogenic versus physiogenic in origin is not productive because the psychogenic view does not carefully consider the limitations of conventional neuroimaging techniques in detecting subtle brain injuries in mTBI, and the physiogenic view does not carefully consider the fact that PTSD and depression, and other co-morbid conditions, may be present in those suffering from mTBI. Further, patients with mTBI may complain more when their symptoms are not validated. That is, when there is no radiological evidence that explains their symptoms, and yet they still experience symptoms, these patients may complain more because of the lack of validation, versus those patients who have radiological evidence that validates their symptoms, leading them to complain less, simply because they have a medical explanation for their symptoms.
The Challenge
The challenge then is to use neuroimaging tools that are sensitive to DAI/TAI, such as Diffusion Tensor Imaging (DTI), to detect brain injuries in mTBI. Specifically, with recent advances in imaging such as DTI it will now be possible to characterize better extant brain injuries in mTBI. Of note, DTI is a relatively new neuroimaging technique that is sensitive to subtle changes in white matter fiber tracts and is capable of revealing microstructural axonal injuries (Basser et al., 1994; Pierpaoli and Basser, 1996; Pierpaoli et al., 1996), which are also potentially responsible for persistent postconcussive symptoms.
Other promising techniques include susceptibility weighted imaging (SWI), which is sensitive to micro- hemorrhages that may occur in mTBI (e.g., Babikian et al., 2005; Haacke et al., 2004; Park et al., 2009; Scheid et al., 2007), and Magnetic Resonance Spectroscopy (MRS), which measures brain chemistry sensitive to neuronal injury and DAI (e.g., Babikian et al., 2006; Brooks et al., 2001; Garnett et al., 2000; Holshouser et al., 2005; Lin et al., 2005; Lin et al., 2010; Provencher 2001; Ross et al., 1998; Ross et al., 2005; Seeger et al., 2003; Shutter et al., 2004; Vagnozzi et al., 2010). In this review we focus primarily on MRI and, most particularly, on DTI findings in mTBI. In a separate article in this special issue, Dr. Alexander Lin and colleagues review MRS, single photon emission tomography (SPECT), and positron emission tomography (PET) findings relevant to brain chemistry alterations in mTBI, and Dr. Brenna McDonald and colleagues review functional MRI (fMRI) findings in mTBI. The reader is also referred to Dr. Robert Stern and colleagues’ article, also in this issue, which reviews the evidence for repetitive concussive and subconcussive injuries in the etiology of chronic traumatic encephalopathy in sports-related injuries such as professional football (see also Stern et al., 2011).
Focus of this Review
Here we present evidence for brain abnormalities in mTBI based on studies using advanced MRI/DTI neuroimaging techniques. Importantly, these advances make it possible to use more sensitive tools to investigate the more subtle brain alterations in mTBI. These advances will likely lead to the development of biomarkers of injury, as well as to staging of reorganization and reversal of white matter and gray matter changes following injury, and to the ability to chart the progression of brain injury over time. Such tools will also likely be used in future research to evaluate treatment efficacy, given their enhanced sensitivity to alterations in the brain.
Taken together, the findings presented below suggest that more sensitive neuroimaging tools improve the detection of brain injuries in mTBI (i.e., diagnosis). These tools will, in the near future, likely provide important information relevant to outcome (prognosis), as well as play a key role in longitudinal studies that are needed to understand the dynamic nature of brain injury in mTBI. We also believe that the enhanced sensitivity of newer and more advanced neuroimaging techniques for identifying brain pathology in mTBI will be important for documenting the biological basis of persistent postconcussive symptoms, which are likely associated with subtle brain alterations, alterations that heretofore have gone undetected due to the lack of sensitivity of earlier, conventional neuroimaging techniques.
Below we provide a brief primer of neuroimaging techniques, although the reader is referred to Kou et al. (2010), Johnston et al. (2001), Le et al. (2009), and Niogi et al. (2010) for more detailed information. For a description of the molecular pathophysiology of brain injury, the reader is referred to Barkhoundarian et al. (2011). The reader is also referred to Dr. Erin Bigler’s article in this special issue for information regarding post-mortem and histological findings in mTBI as well as for a discussion of the physiological mechanisms underlying TBI. Dr. Bigler emphasizes that neuroimaging abnormalities are “gross indicators” of the underlying cellular damage resulting from trauma-induced pathology. We concur and believe that we now have neuroimaging tools that are sufficiently sensitive to discern both more gross indicators of pathology, as well as microstructural changes in white matter, and micro-hemorrhages using newer imaging technologies. The reader is also referred to Smith et al. (1995) and to several recent and excellent reviews of neuroimaging findings in mTBI (e.g., Belanger et al., 2007; Bruns and Jagoda 2009; Gentry, 1994; Green et al., 2010; Hunter et al., 2011; Kou et al., 2010; Le et al., 2009; Maller et al., 2010; Niogi et al., 2010). Jang (2011) has also published a recent review of the use of DTI in evaluating corticospinal tract injuries after TBI.
Following the brief primer, we present MRI and DTI findings relevant to mTBI. We used PUBMED to locate these articles. The following keywords were used: (MRI or DTI or Diffusion Tensor) AND (Concussion or Mild TBI or Mild Traumatic Brain Injury or mTBI). The dates for the articles selected were inclusive to September 16, 2011. We did not include articles that were case studies, nor did we include articles that focused on pediatric and adolescent populations (see article in this special issue by Wilde and colleagues, which covers this topic). We also did not include articles that did not specify the severity of injury, but instead described only the mechanism of injury, i.e., falls, motor vehicle accident, hit by tram (e.g., Liu et al., 1999). For the morphometric MRI empirical studies, we note that most included mild, moderate, and severe TBI, rather than mTBI alone. Consequently we included all three. This was less the case for the DTI empirical studies, where many focused only on mTBI. We were thus able to separate empirical studies that focused solely on mTBI from those that included several levels of severity, although we report on both. We include detailed summary tables of MRI and DTI findings in order to provide the interested reader with a more in depth and detailed review of each empirical study included in this review. Following the review of MRI and DTI findings, we present future directions for research in mTBI, which include the use of multiple modalities for imaging the same patients, and the importance of following patients longitudinally. We also present new imaging methods that go beyond advanced imaging approaches reviewed here that, to date, are still as yet not used routinely in a clinical setting. The potential for developing biomarkers to identify and to characterize mTBI is also presented. The need here is critical as mTBI is not only difficult to detect but the injuries to the brain are heterogeneous, and biomarkers are needed for individualized diagnosis as well as for early and effective treatment interventions.
Neuroimaging Primer and Role of Neuroimaging in mTBI
Overview
TBI is a heterogeneous disorder and there is no one single imaging modality that is capable of characterizing the multifaceted nature of TBI. Advances in neuroimaging are, nonetheless, unprecedented and we are now able to visualize and to quantify information about brain alterations in the living brain in a manner that has previously not been possible. These advances began with computed axial tomography (CT) in the 1970’s, and then with magnetic resonance imaging (MRI) in the mid-1980’s, with more refined and advanced MR imaging over the last 25 years, including perfusion weighted imaging (useful for measuring abnormal blood supply and perfusion), susceptibility-weighted imaging (SWI; useful for measuring micro-hemorrhages – e.g., Haacke et al., 2004; Park et al., 2009), magnetization transfer MRI (useful for measuring traumatic lesions – e.g., see review in Le et al., 2009), diffusion weighted imaging (DWI; useful for measuring edema and developed initially for studies of stroke – see review in Le et al., 2009), diffusion tensor imaging (DTI; useful for measuring white matter integrity – e.g., Basser et al., 1994), and functional MRI (fMRI; useful for measuring altered cortical responses to controlled stimuli – e.g., see article by McDonald et al. in this issue). Other neuroimaging tools, although not a complete list, include positron emission tomography (PET; useful for measuring regional brain metabolism using 2-fluro-2-deoxy-d-glucose, both hyper and hypo metabolism observed in TBI – see Le et al., 2009 for review), single photon emission tomography (SPECT; useful for measuring cerebral blood flow but less sensitive to smaller lesions that are observed on MRI – see article by Lin et al. in this issue), and magnetic resonance spectroscopy (MRS; useful for measuring brain metabolites/altered brain chemistry – see article by Lin et al. in this issue). The clinical use of such tools lags behind their development, although the gap between development and clinical application is narrowing.
Below, we provide a brief primer for some of the neuroimaging tools available today. We include skull films, CT, and MRI including DWI/DTI, and susceptibility weighted imaging. This primer is not detailed nor is it comprehensive. Instead, our intention is to provide the reader who is less familiar with neuroimaging techniques with a context for some of the tools available for investigating mTBI. Other neuroimaging modalities, which will not be described here, include MRS, PET, SPECT, and fMRI. MRS, PET, and SPECT, will be reviewed by Dr. Alexander Lin and colleagues, and Dr. Brenna McDonald and colleagues will review fMRI, in separate articles in this issue. Table 1 provides a brief summary of these neuroimaging tools.
Table 1.
Summary of Modalities
| Imaging Technique/Modality: | Function: | Advantages Offered: |
|---|---|---|
| X-ray | Imaging of bony structures | Primarily used for detecting fractures |
| Computed Tomography (CT) | 3D X-ray imaging of an object (e.g., brain and skull). | Quick, able to have medical equipment in scanning area, good for skull fractures or gross injuries/abnormalities requiring emergent surgical intervention such as subdural hematomas. |
| Clinical Magnetic Resonance Imaging (MRI) | Uses radiofrequency pulses to detect changes in spin signal of hydrogen atoms. | Better resolution than CT, particularly for soft tissue, can provide gross delineation between gray and white matter structures, better visualization of brain stem areas compared to CT, can also detect subacute hemorrhages and macroscopic areas of white matter damage. |
| Diffusion Weighted Imaging (DWI)/Diffusion Tensor Imaging (DTI) | Special type of MRI sequence that uses the diffusion properties of water to detect microstructural tissue architecture. | Best imaging technique available for detecting white matter integrity/damage, able to detect microscopic white matter damage and trace specific tracts of the brain (e.g., corpus callosum, superior longitudinal fasciculus, uncinate). |
| Susceptibility Weighted Imaging (SWI) | Special type of MRI technique that takes advantage of susceptibility differences among structures (e.g. oxygenated vs. deoxygenated blood and iron). | Provides increased sensitivity to detect areas of micro-hemorrhage, particularly at gray-white matter junctions, that are not detectable on standard MRI. |
| Magnetic Resonance Spectroscopy (MRS) | Measures brain chemistry by producing a spectrum where individual chemicals, or metabolites can be identified and concentrations can be measured. | Provides neurophysiological data that is related to structural damage/changes, neuronal health, neurotransmission, hypoxia, and other brain functions |
| Positron Emission Tomography (PET) | Uses radiotracers labeled with different isotopes that emit signals indicating areas of uptake or binding in the brain, most commonly used is 18-Fluorodeoxyglucose, an analog of glucose. | Provides information on the concentration of a chemical or protein in the brain such as the amount of glucose, which reflects activity, or the density of a type of protein such as beta amyloid, a hallmark of neurodegenerative disease. |
Skull-X-ray and CT
Skull films, or skull X-rays, while excellent for detecting skull fracture, are not used routinely to investigate brain trauma because they provide very limited information (e.g., Bell et al., 1971; Hackney, 1991). Figure 1 depicts a normal skull film. Computed Tomography or Computed Axial Tomography (CT) supplanted the use of skull films for evaluating neurotrauma when this technology became available in the 1970s. CT provides three-dimensional images of the inside of an object, in this case the brain, using two-dimensional X-Ray images obtained around a single axis of rotation. Since CT was introduced in the 1970s, it has become the imaging modality of choice for evaluating closed head injury in the emergency room (ER) (e.g., Johnston et al., 2001).
Figure 1.
Lateral (left) and frontal (right) view of normal skull X-ray. (Courtesy of Amir Arsalan Zamani, M.D.)
CT is, in fact, the main imaging modality used in the first 24 hours for the management of neurotrauma in the ER (e.g., Coles et al., 2007). The reasons for this are because it is widely available in most hospitals, it is fast, and it is accurate for detecting emergent conditions such as skull fractures, brain swelling, intracranial hemorrhage, herniation, and radio-opaque foreign bodies in the brain (see review in Johnston et al., 2001; Le et al., 2009). The use of thin-volume CT scanners are also often located in close physical proximity to the ER, thus making it easy to transport neurotrauma patients. Additionally, the presence of metallic objects will not result in possibly dangerous accidents in the CT suite as would be the case using an MR scan, depending upon the nature of the trauma, and depending upon whether or not unknown small pieces of metal are hidden inside the patient following a car accident or other type of brain trauma. Of further note, MRI scanners are generally not in close physical proximity to the ER, and the scanning time is longer, which is an important consideration for patients who are not medically stable. Moreover, the CT environment is able to accommodate the set up of life support and monitoring equipment that is, at this time, often more compatible for the CT than for the MRI environment, although this is changing. CT thus remains the most important neuroimaging tool used in the first 24 hours of acute neurotrauma in the ER, where the most important question to be answered quickly is: does this person need immediate neurosurgical intervention?
Figure 2 depicts a normal CT scan. Note that the skull and the brain are visible, although there is no differentiation between gray and white matter, which is discernible using MRI. There are also bone artifacts with CT that are not present with MRI, which means that areas of injury around bone are easier to detect using MRI. MRI also uses no ionizing energy, as CT does, which becomes important when considering pediatric populations. This is also a consideration when several repeat scans are needed over time to follow the progression of injury.
Figure 2.
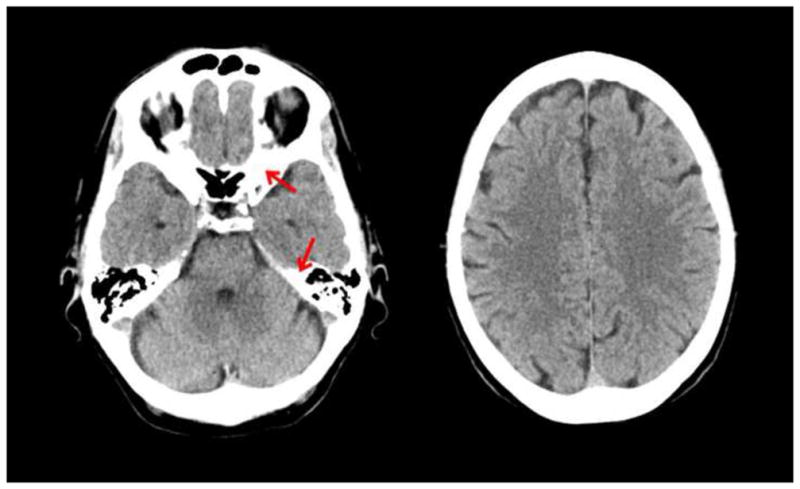
CT scan of a normal brain. Left side is at the level of the temporal lobe where bone can be seen as white areas (see red arrows). Right side is at the level of the frontal lobe. (Courtesy of Amir Arsalan Zamani, M.D.)
MRI and SWI
Magnetic resonance imaging was introduced in the mid-1980s with the first images acquired on low-field magnets, i.e., 0.5 Tesla (T). Originally this type of imaging was called nuclear magnetic resonance (NMR) imaging but the name was changed to magnetic resonance (MR) imaging, or MRI. The basic principle behind MRI is that radiofrequency (RF) pulses are used to excite hydrogen nuclei (single proton) in water molecules in the human body, in this case the brain. By modulating the basic magnetic field, and the timing of a sequence of RF pulses, the scanner produces a signal that is spatially encoded and results in images. While NMR can be observed with a number of nuclei, hydrogen imaging is the only one that is widely used in the medical use of MRI.
MR images can be produced with different contrasts and can be optimized to show excellent contrast between gray and white matter, which CT does not. Early MRI scans had poor spatial resolution and the time to acquire images was slow, taking many minutes to acquire even one image. Since the mid-1980s, however, the field strengths of the magnet have increased from 0.5 to 1.0, to 1.5T, and to 3.0T and beyond. In combination with advances in the capabilities of the gradient magnetic fields and the RF equipment available (parallel imaging), it is now possible to acquire sub-millimeter morphologic images and rich contrast combinations in clinical settings, in a shorter period of time. Moreover, reconstruction algorithms can recreate images even when the volume of the pixel elements (voxels) is not completely isotropic (i.e., the same size in all directions). Figure 3 depicts MRI scans acquired on a 3T magnet using 1.5mm slices. Note the high contrast between gray and white matter that is not visible on CT (see Figure 2). Cerebral spinal fluid (CSF) is also prominent, and one can use the differences in signal intensity of gray matter, white matter, and CSF to parcellate automatically the brain into these three tissue classes (e.g., Fischl et al. 2004; Pohl et al., 2007). Of note here, the different tissue classes, from the parcellation, include quantitative information such as whole brain volume for gray matter, white matter, and CSF. This work is based on research developed over more than a decade in the field of computer vision.
Figure 3.
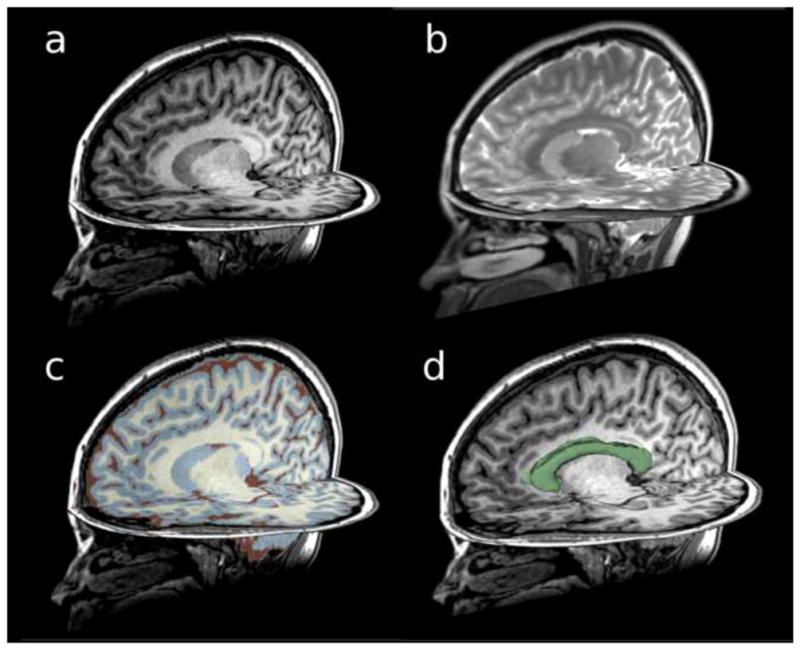
Structural MRI scans acquired on a 3T magnet using 1.5mm slices: (a) T1-weighted image, (b) T2-weighted image, (c) T1-weighted image showing gray matter, white matter, and CSF parcellation, and (d) T1-weighted image showing the corpus callosum region of interest.
Due to its superior contrast resolution for soft tissues, MRI technology is far more sensitive than CT in detecting small contusions, white matter shearing, small foci of axonal injury, and small subacute hemorrhages (see review in Niogi et al., 2010). That MRI is able to discern these more subtle abnormalities, compared with CT, makes it particularly well suited for evaluating mTBI. Additionally, there is higher contrast between brain and CSF, and between gray and white matter, as well as better detection of edema with MRI than CT, all important factors in evaluating TBI (see review in Johnston et al., 2001).
Of further note, and of particular interest to mTBI, Mittl and coworkers (1994) found that in mTBI, where CT findings were negative, 30% of these cases showed lesions on MRI that were compatible with hemorrhagic and non-hemorrhagic diffuse axonal injuries. The increased sensitivity of MRI over CT in discerning radiological evidence of brain injury in mTBI has also been shown, and commented upon, by a number of other investigators including Jenkins and coworkers (1986), Levin and coworkers (1984, 1987), Eisenberg and coworkers (1989), and Bazarian and coworkers (2007). Gentry and coworkers (1988) also observed that in a prospective study of 40 closed injury patients, MRI was superior to CT in detecting non-hemorrhagic lesions. These findings, taken together, suggest that while CT may be critically important in the first 24 hours to assess the immediate need for neurosurgical intervention, for mTBI, MRI is likely to be more sensitive for detecting small and subtle abnormalities that are not detected using CT (e.g., Gentry et al., 1988; Levin et al., 1987).
There are also several types of MRI sequences that add to what can be gleaned from conventional MRI, including the use of T1, T2-weighted FLAIR (FLuid Attenuated Inversion Recovery) to examine macroscopic white matter lesions and contusions on the cortical surface, as well as susceptibility-weighted imaging (SWI), which is a type of gradient-recalled echo (GRE) MRI that can be performed on conventional scanners. SWI was originally developed for venography and called Blood-Oxygen-Level-Dependent (BOLD) venographic imaging (Ashwal et al., 2006; Haacke et al., 2009; Reichenbach et al., 2000; see also review in Kou et al., 2008 and Niogi et al., 2010). SWI takes advantage of susceptibility differences between tissues, resulting in an enhanced contrast that is sensitive to paramagnetic properties of intravascular deoxyhemoglobin, i.e., sensitive to venous blood, to hemorrhage, and to iron in the brain. In essence, susceptibility differences are detected as phase differences in the MRI signal. In the image processing stage, SWI superimposes these phase differences on the usual (magnitude) MR image, thereby allowing the susceptibility differences to be accentuated in the final image. Of further note, SWI shows six times greater ability to detect hemorrhagic diffuse axonal injuries than other MRI techniques (Tong et al., 2003; 2004). This technique is thus particularly appropriate for discerning micro-hemorrhages in TBI, as it is sensitive to bleeding in gray/white matter boundaries, where small and subtle lesions are not discernible using other MRI techniques, making it particularly useful in the more acute and subacute stages following brain trauma. SWI, in conjunction with diffusion measures (e.g., DTI), will thus likely be important for discerning the subtle nature of mTBI abnormalities in the future. SWI is offered as a licensed acquisition and processing package by several vendors, but it can be acquired and processed on any scanners that are 1.0T, 1.5T, 3.0T, or above. Figure 4 depicts susceptibility-weighted images, where small black areas indicate blood vessels.
Figure 4.
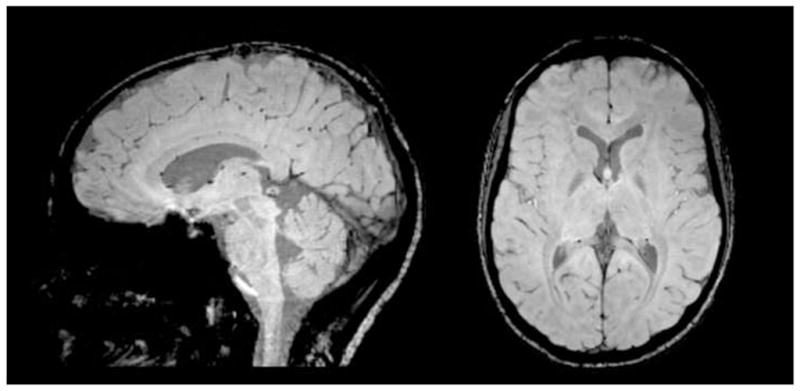
Sagittal (left) and axial (right) view of susceptibility-weighted images (SWI) of a normal brain. Small black areas indicate blood vessels in the brain that are enhanced using SWI.
DWI and DTI
Diffusion weighted imaging (DWI), developed in 1991 for use in humans (e.g., LeBihan et al., 1991), is based on the random motion of water molecules (i.e., Brownian motion). This motion in the brain is affected by the intrinsic speed of water displacement depending upon the tissue properties and type, i.e., gray matter, white matter, and CSF. DWI was first used to evaluate acute cerebral ischemia where it was thought that decreased diffusion was the result of neuronal and glial swelling and likely related to cytotoxic edema, whereas increased diffusion was thought to reflect vasogenic edema. The method has been applied to TBI with mixed results (see Niogi et al., 2010).
Apparent Diffusion Coefficient (ADC) is a measure of diffusion, on average, and the word “apparent” is used to emphasize that what is quantified is at the level of the voxel, and not at the microscopic level. This measure has been used as an indicator of edema, which, in conjunction with DTI (see below), can be used to quantify, indirectly, both edema and damage to the integrity of white matter fiber bundles in TBI (see review in Assaf and Pasternak, 2008; Niogi et al., 2010). A measure of free water, however, derived from DTI (Pasternak et al., 2009; 2010; 2011a&b) may provide a better measure of edema, and this will be discussed further in the section on future directions of research.
DTI is a DWI technique that has opened up new possibilities for investigating white matter in vivo as it provides information about white matter anatomy that is not available using any other method — either in vivo or in vitro (Basser et al., 1994; Pierpaoli and Basser, 1996; Pierpaoli et al., 1996; see also review in Assaf and Pasternak, 2008). At today’s image resolution, it does not detect water behavior within individual axons. Instead it describes local diffusion properties. In other words, the individual behavior of axons cannot be described using DTI, but diffusion properties can be described that are relevant to fiber bundles.
DTI differs from conventional MRI in that it is sensitive to microstructural changes, particularly in white matter, whereas CT and conventional MRI (including also FLAIR) reveal only macroscopic changes in the brain. Thus subtle changes using DTI can reveal microstructural axonal injuries (Basser et al., 1994; Pierpaoli and Basser, 1996; Pierpaoli et al., 1996), which are potentially responsible also for persistent postconcussive symptoms.
The concept underlying DTI is that the local profile of the diffusion in different directions provides important indirect information about the microstructure of the underlying tissue. It has been invaluable in investigations of white matter pathology in multiple sclerosis, stroke, normal aging, Alzheimer’s disease, schizophrenia and other psychiatric disorders, as well as in characterizing diffuse axonal injuries in mTBI (see reviews in Assaf and Pasternak, 2008; Kou et al., 2010; Shenton et al., 2010; Whitford et al., 2011).
The latter focus on TBI is relatively recent (see review of the literature, below). Those investigating mTBI, in particular, have been disappointed by the lack of information gleaned from conventional MRI and CT, although, as noted previously, this is not surprising given that the most common injuries observed in mTBI are diffuse axonal injury/traumatic axonal injury (DAI/TAI), which are not easily detected using conventional MR or CT scans. With the advent of DTI, however, DAI/TAI have the potential to be quantified and this information can be used for diagnosis, prognosis, and for the evaluation of treatment efficacy.
Quantification of pathology using DTI is based on measures that calculate the amount of restriction of water movement in the brain, which is determined to a large extent by the tissue being measured. For example, the movement of water is unrestricted in a medium such as CSF, where it diffuses equally in all directions (i.e., isotropic). However, in white matter, the movement of water is more restricted by axonal membranes, myelin sheaths, microtubules, neurofilaments, etc. In white matter, this restriction is dependent on the directionality of the axons (i.e., diffusion is not equal in all directions) and is referred to as anisotropic diffusion.
Using tensors, adapted from the field of engineering, the average shape of the diffusion is characterized as more or less spherical when there is no impediment to water diffusion, as for example in CSF (i.e., unrestricted water is free to diffuse in all directions: isotropic). However, the average shape of the diffusion becomes more elongated, or cigar shaped, when there is a preferred orientation in which water is restricted, as for example in white matter. Here, water diffuses freely in directions parallel to axons but it is restricted in directions that are perpendicular to the axons, which results in the magnitude of the diffusion along the axons being larger than the two perpendicular directions, leading to an elongated ellipsoidal shape of the diffusion tensor, described as anisotropic. The measurement of the distance that water diffuses, over a given period of time, for at least six non-collinear directions, makes it possible to reconstruct a diffusion tensor (and the associated ellipsoid) that best describes water diffusion within a given voxel. Consequently, the volume (size) and shape of the ellipsoid can be calculated, and this provides important information about the diffusion properties, and hence about microstructural aspects of brain tissue.
There are various ways that the shape and size of a diffusion ellipsoid can be quantified, but the two most common indices used are Fractional Anisotropy (FA) for shape, and Mean Diffusivity (MD) for size. FA is a scalar measure that ranges from 0 to 1, with 0 being completely isotropic, meaning that water diffuses equally in all directions, and 1 depicting the most extreme anisotropic scenario in which molecules are diffusing along a single axis. Accordingly, in CSF and gray matter, as noted above, the direction of water is equal in all directions (i.e., isotropic), and the value is close to 0. In contrast, in white matter, for example in the corpus callosum, the water is relatively free along the axons, but restricted perpendicular to the axons, and therefore more anisotropic, with FA being closer to 1. Thus in white matter, reduced FA is generally thought to reflect loss of white matter integrity that may reflect damage to myelin or axon membrane damage, or perhaps reduced axonal packing density, and/or reduced axonal coherence (see review in Kubicki et al., 2007).
Mean diffusivity (MD), the second most common measure (and proportional to the trace of the diffusion tensor), is different from FA in that it is a measure of the size of the ellipsoid, rather than the shape, as is the case for FA. MD is similar to ADC, described above for DWI, but instead it is the average ADC along the three principal diffusion directions, where one axis is in the direction of the largest magnitude of the diffusion in the voxel, and the other two are perpendicular to the main diffusion direction. The main diffusion direction in white matter is referred to as the longitudinal or axial direction, while the other two directions are referred to as the radial or tangent axes. FA and MD are frequently observed as being inversely related. (For further descriptions of DTI and associated methods of analyses, the reader is referred to Pierpaoli and Basser, 1996; Pierpaoli et al., 1996; Smith et al., 2006; and the reviews in Ashwal et al., 2006; Fitzgerald et al., 2011; Hunter et al., 2011; Le et al., 2009; Kou et al., 2010; and Niogi et al., 2010).
Figure 5, 6, and 7 depict the kind information that can be extracted from diffusion tensor images. For example, Figure 5 shows diffusion images that highlight white matter, along with colored maps that reflect the directions of the white matter fiber tracts in the brain. Figure 6 shows white matter tracts superimposed on structural images. Figure 7 shows an area identified as tumor in the frontal lobe, where white matter fiber tracts can be visualized in relation to the tumor and in relation to the frontal horn of the lateral ventricles. These figures reflect important, recent advances in methodology that are sufficiently robust and sensitive that they can be used for visualizing and quantifying white matter pathology in vivo, for the assessment of mTBI clinically. These tools are available now for this purpose and will be discussed further in the future directions section of this article.
Figure 5.
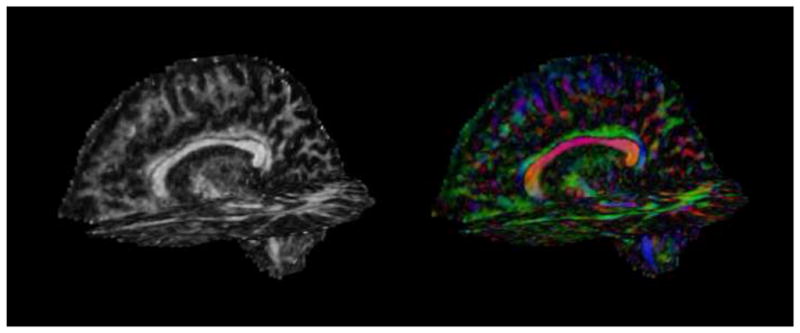
Diffusion tensor images acquired on a 3T magnet. Left: fractional anisotropy (FA) map. White areas are areas of high anisotropy. Right: color by orientation map. Diffusion in the left-right direction is shown in red, diffusion in the superior-inferior direction is shown in blue, and diffusion in the anterior-posterior direction is shown in green.
Figure 6.
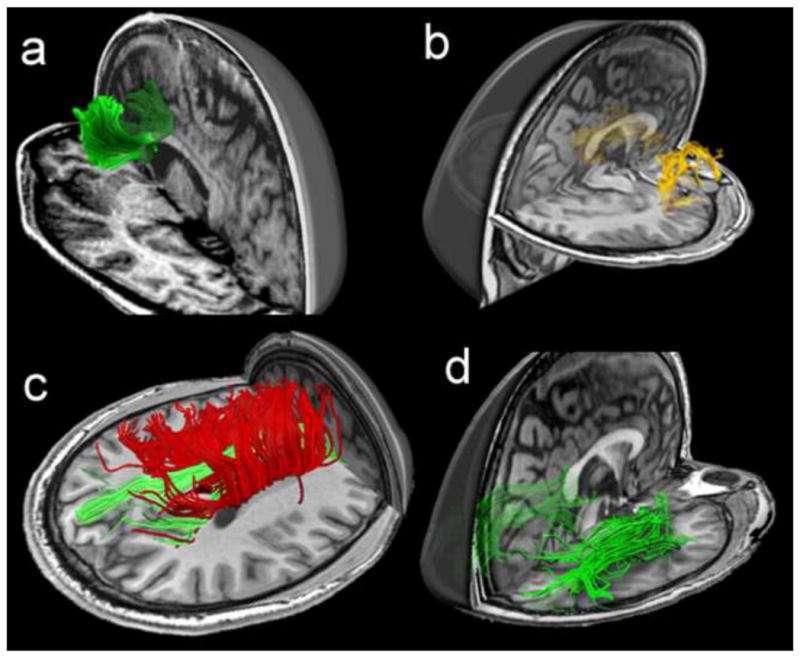
Fiber tractography of commonly damaged tracts in mild traumatic brain injury, including: (a) the anterior corona radiata and the genu of corpus callosum, (b) the uncinate fasciculus, (c) the cingulum bundle in green and the body of corpus callosum in red, and (d) the inferior longitudinal fasciculus (Niogi et al., 2010; reprinted with permission Wolters Kluwer Health/Lippincott Williams & Wilkins)
Figure 7.
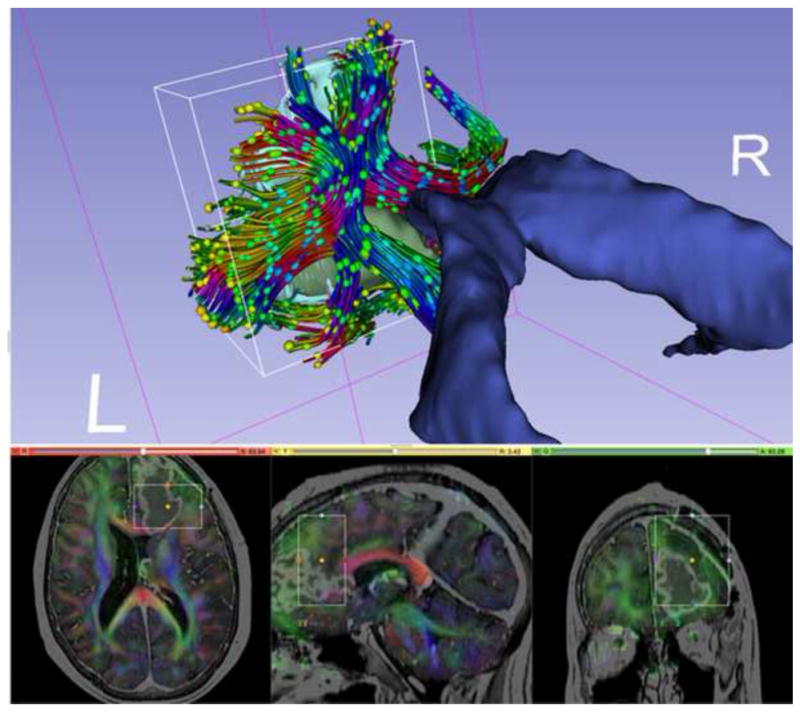
Diffusion MRI data for neurosurgical planning. The tractography region of interest (ROI) is a box placed around the tumor (in green) in the frontal lobe. The ROI is also visualized with rectangles in the slice views below. Tracts are then created based on the principal diffusion directions, which are color-coded (bottom). Diffusion ellipsoids are shown along the tract to visualize the shape of the local diffusion.
DTI, however, is somewhat non-specific and it is not known whether disruptions in FA and MD are the result of disturbances in axonal membranes, myelin sheath, microtubules, neurofilaments, or other factors. More specific measures, which are being developed (see below), are needed to delineate further the biological meaning of alterations in white matter integrity (see review in Assaf and Pasternak, 2008; Niogi et al., 2010).
While FA and MD are the two main dependent measures derived from DTI, there are other measures that have been developed, including Mode (Ennis et al., 2006), which defines more precisely the shape of the diffusion tensor (useful in distinguishing the anatomy of fiber tracts, including distinguishing fiber crossings from pathology). Other measures include Inter-Voxel Coherence (Pfefferbaum et al., 2000), which measures how similar anisotropic tensors are in neighboring voxels, useful in measuring anomalies in macroscopic axonal organization within the tract of interest, and Axial and Radial Diffusivity, which are purported to measure axonal and myelin pathology, respectively (Song et al., 2001; Song et al., 2003; Budde et al., 2007; Budde et al., 2011). These additional measures may provide more specific information regarding the microstructural abnormalities discerned using the sensitive, albeit less specific, measures of FA and MD.
Finally, another relatively new post-processing method is fiber tractography, which was developed to visualize and to quantify white matter fiber bundles in the brain (e.g., Conturo et al., 1999; Mori et al., 1999; Basser et al., 2000). This method makes it possible to follow fiber tracts along a diffusion direction in very small steps so as to create long fiber tracts that connect distant brain regions. The accuracy of fiber tractography is dependent upon a number of factors including image resolution, noise, image distortions and partial volume effects that result from multiple tracts crossing in a single voxel. The main advantage of DTI tractography, from a clinical research perspective, is that the whole fiber bundle, instead of just a portion of the fiber bundle, can be evaluated. DTI tractography is thus a promising tool that can be used not only to understand how specific brain regions are connected and where damage occurs along fiber bundles, but it can also be used to understand how this connectivity may be relevant to functional abnormalities. Further, tractography methods can be used to both visualize and to quantify white matter fiber bundle damage in a single case and thus these methods are potentially important for diagnosing mTBI based on radiological evidence.
Importantly, many of the measures described above are just beginning to be applied to investigate brain injuries in mTBI and thus this area is a relatively new frontier for exploration. The application of DTI, and the measures derived from DTI, will likely contribute enormously to our understanding of the nature and dynamics of brain injuries in mTBI.
Review of MRI Findings in mTBI
Much of the work with MRI has been to investigate the higher sensitivity of MRI, compared with CT, for detecting brain abnormalities in mTBI (see previous discussion). Less attention has been given to investigating morphometric abnormalities in mTBI using area, cortical thickness, and/or volume measures. Table 2 lists studies, by first author and year, which have examined aspects of morphometric abnormalities in patients with mTBI. Most of these studies, however, include a range of TBI, from mild to severe (e.g., Anderson et al., 1995; Anderson et al., 1996; Bergerson et al., 2004; Bigler et al., 1997; Ding et al., 2008; Fujiwara et al., 2008; Gale et al., 2005; Levine et al., 2008; Mackenzie et al., 2002; Schonberger et al., 2009; Strangman et al., 2010; Tate et al., 2000; Trivedi et al., 2007; Warner et al., 2010a&b; Wilde et al., 2004; Wilde et al., 2006; Yount et al., 2002), with only a small number of studies that investigate morphometric abnormalities specifically in mTBI (e.g., Cohen et al., 2007; Holli et al. 2010). Additionally, while most of the studies listed in Table 2 categorize severity of TBI (i.e., mild, moderate, or severe) based on scores derived from the Glasgow Coma Scale (GCS; Teasdale and Jennett, 1974), one study defines severity by posttraumatic amnesia (PTA) duration (Himanen et al., 2005).
Table 2.
MR Morphometry Studies
| First Author | Year | Time Post-Injury | Magnet | Subjects | Analysis Method | Main Findings |
|---|---|---|---|---|---|---|
| Anderson | 1995 | Subacute. ≥ 6 weeks. |
Not Specified |
Patients: 68 TBI patients (49M, 19F), [GCS 3–15]; 38 with frontal lesions and 29 with no frontal lesions. Controls: None. |
Volumetric Analysis. |
|
| Gale | 1995 | Chronic. Average 21 months. |
1.5T |
Patients: 88 TBI patients (51M, 37F; mean age 28.5), [GCS 3–15], 36 mTBI [GCS 11–15], 22 moderate TBI [GCS 7–10], 29 severe TBI [GCS 3–6]. Controls: 73 controls (36M, 37F; mean age 31). |
Volumetric Analysis. |
|
| Anderson | 1996 | Subacute and Chronic. ≥ 6 weeks. |
Not Specified |
Patients: 63 TBI patients (45M, 18F); 35 with lesions (26M, 9F; mean age 29.43), 28 without lesions (19M, 9F; mean age 31.33). Controls: 33 controls (26M, 9F; mean age 29.24). |
Volumetric Analysis. |
|
| Bigler | 1997 | Subacute and Chronic. 44 scans ≤ 100 days post-injury, 55 scans > 100 days post-injury. |
1.5 |
Patients: 94 TBI patients (59M and 35F; mean age 27) [GCS 3–15]. Controls: 96 controls (37M, 59F; mean age 31). |
Volumetric Analysis. |
|
| Tate | 2000 | Subacute and Chronic. ≥ 2 months. |
1.5 |
Patients: 86 TBI patients (58M, 28F; mean age 30.9), [GCS 3–15]. Controls: 46 controls (31M, 15F; mean age 37.21). |
Volumetric Analysis. |
|
| MacKenzie | 2002 | Subacute and Chronic. 14 patients at >3 months after injury, 7 patients at 2 time points >3 months apart. |
1.5T |
Patients: 14 patients (mean age 36.1) [GCS 9–15]; 11 with mTBI [GCS 13–15], and 3 with moderate TBI [GCS 9–12]. Controls: 10 controls (mean age 34.9) underwent one MR session, and 4/10 underwent 2 sessions > 3 months apart. |
Volumetric Analysis. |
|
| Yount | 2002 | Chronic. Average 22.8 months. |
1.5T |
Patients: 27 patients (18M, 9F; mean age 26) with TBI, [GCS 4–14]. No focal lesions or infarctions on MRI. Controls: 12 age and gender-matched controls. |
Volumetric Analysis. |
|
| Bergeson | 2004 | Subacute and Chronic. ≥ 90 days. |
1.5 T |
Patients: 75 TBI patients (50M, 25 F; mean age 32.9) [GCS 3–14]. Controls: 75 controls (50M, 25F; mean age 31.4). |
Volumetric Analysis. |
|
| Wilde | 2004 | Subacute and Chronic. ≥ 90 days. |
1.5T |
Patients: 77 patients [GCS 3–15]; 25 TBI and positive blood alcohol level (BAL) and 52 TBI with negative BAL. Controls: None. |
Volumetric Analysis. |
|
| Gale | 2005 | Chronic. Approximately 1 year (mean 10.6 months). |
1.5T |
Patients: 9 patients with a history of TBI (8M, 1F; mean age 29.1), [GCS 5–15]. Controls: 9 controls: (8M, 1F; mean age 28.8). |
Voxel Based Morphometry. |
|
| Himanen | 2005 | Chronic. Average 30 years. |
1.5T |
Patients: 61 patients (41M, 20F; mean age at injury 29.4); 17 mTBI [post-traumatic amnesia (PTA) <1 hr], 12 moderate TBI [PTA 1–24 hrs], 11 severe [PTA 1–7 days], 21 very severe [PTA >7 days] [GCS not reported]. Controls: None. |
Volumetric Analysis. |
|
| Wilde | 2006 | Suacute and Chronic. ≥ 90 days. |
1.5 T |
Patients: 60 patients with severe-to-mild [GCS 3–15] TBI (38 M, 22 F; mean age 28.6). Controls: None. |
Volumetric Analysis. |
|
| Cohen | 2007 | Acute to Chronic. 7 patients within 9 days, 13 patients from 1.2 months to 31.5 (average 4.6 years). |
1.5T |
Patients: 20 mTBI patients (11 M, 9F; median age 35), [GCS 13–15]. Controls: 19 controls (11M, 8F; median age 39). |
Lesion Detection and Volumetric Analysis. |
|
| Trivedi | 2007 | Subacute. Approximately 79 days; rescanned at approximately 409 days. |
3T |
Patients: 37 TBI patients (27M, 9F, 1 N/A; mean age 29.3); 11 mTBI [GCS ≥13], 10 moderate TBI [GCS 9–12], 16 severe TBI [GCS ≤8]. Controls: 30 controls (13M, 7F; mean age 24.5). |
Volumetric Analysis. |
|
| Ding | 2008 | Acute and Chronic. (≤ 1 month) and (≥ 6 months). |
3T |
Patients: 20 patients with TBI (13M, 7F; mean age 26); 4 complicated-mild TBI [GCS ≥13], 4 moderate TBI [GCS 9–12]; and 12 severe TBI [GCS ≤8]. Controls: 20 controls (13M, 7F; mean age 28) |
FLAIR Lesion Volume and Volumetric Analysis. |
|
| Fujiwara | 2008 | Chronic. Approximately 1 year. |
1.5T |
Patients: 58 TBI patients underwent MRI; 12 mild [GCS 13–15], 27 moderate [GCS 9–12], and 19 severe [GCS 3–8]. 18 patients with focal cortical contusions and 40 with diffuse injury only. Controls: 25 controls; behavioral testing only. |
Semi-Automated Segmentation (SABRE). |
|
| Levine | 2008 | Chronic. ≥ 1 year. |
1.5T |
Patients: 69 TBI patients; 13 mild [mean GCS 14.6], 30 moderate [mean GCS 11.1], 26 severe [mean GCS 6.7]. Controls: 12 age- and sex-matched controls. |
Semi-Automated Segmentation (SABRE). |
|
| Schonberger | 2009 | Chronic. Average 2.3 years. |
1.5T |
Patients: 99 patients with mild to severe TBI (74 M, 24 F; mean age 34.5), [GCS 3–15]. Controls: None. |
Lesion Volume, Automated Segmentation and Volumetric Analysis (SPM5). |
|
| Holli | 2010(a) | Acute. ≤ 3 weeks. |
1.5T |
Patients: 42 mTBI patients (17 M, 25F; mean age 38), [GCS 13–15]. All patients had normal clinical CT and MRI. Controls: 10 controls (4M, 6F; mean age 39.8). |
Texture analysis (MaZda). |
|
| Strangman | 2010 | Chronic. Average 11.5 years. |
1.5T |
Patients 50 TBI patients (36M, 14F; mean age 47.2) with TBI with reported memory difficulties; 12 mild [LOC ≤30 min, or GCS 13–15], 12 moderate [LOC 30 min −24 hr or GCS 9–12], 24 severe [LOC >24 hr or GCS <9], 2 n/a. Controls: None. |
Automated Segmentation and Volumetric Analysis (FreeSurfer). |
|
| Warner | 2010a | Acute. Initial median 1 day post-injury. Follow-up at median 7.9 months post-injury. |
3T |
Patients: 25 patients with diffuse traumatic axonal injury (18M, 7F; mean age 26.8), [GCS 3–15]. Controls: 22 Controls (14M, 8F, mean age 32.4). |
Automated Segmentation and Volumetric Analysis (FreeSurfer). |
|
| Warner | 2010b | Acute. Initial ≤ 1 week post-injury. Follow-up at 4–16 months post-injury. |
3T |
Patients: 24 patients with diffuse traumatic axonal injury (16M, 8F; mean age 27.2) [mean GCS 6.4; range not reported]. Controls: None. |
Automated Segmentation and Volumetric Analysis (FreeSurfer). |
|
| Yurgelun-Todd | 2011 | Not Reported. | 3T |
Patients: 15 patients with one or more TBI (all M; mean age 34.9); 14 with at least one mild TBI; 1 with moderate/severe TBI. [GCS not reported; (mild, moderate and severe according to The Ohio State University— TBI Identification Method (OSU-TBI)]. 14 subjects were normal on a clinical MRI. Controls 17 controls (all M; mean age 34.0). |
Automated Segmentation and Volumetric Analysis (FreeSurfer). |
|
Key: M=male; F=female; GCS=Glascow Coma Scale; CC=Corpus Callosum. Acute defined as under 1 month, subacute is defined as >1 month but < 6 months, and chronic is defined as >6 months.
The time of scan post-injury has also varied considerably from study to study with the least amount of time being a median of one day (Warner et al., 2010), up to a mean of 30 years (Himanen et al., 2005), with one study that did not report time of scan post-injury (Yurgelun-Todd et al., 2011). Additionally, most of these studies were performed using a 1.5T magnet, with only a small number performed using a 3T magnet (e.g., Ding et al., 2008; Trivedi et al., 2007; Warner et al., 2010a&b; Yurgelun-Todd et al., 2011). There are also different methods used to evaluate brain injuries, ranging from manual and automated measures of lesion volume (e.g., Cohen et al., 2007; Ding et al., 2008; Schonberger et al., 2009), to volume analysis (e.g., Anderson et al., 1995; Anderson et al., 1996; Bergerson et al., 2004; Bigler et al., 1997; Ding et al., 2008; Gale et al., 1995; Himanen et al., 2005; Mackenzie et al., 2002; Schonberger et al., 2009; Strangman et al., 2010; Tate et al., 2000; Trivedi et al., 2007; Warner et al., 2010a&b; Wilde et al., 2004; Wilde et al., 2006; Yount et al., 2002), to voxel-based-morphometry (VBM; Gale et al., 2005), to texture analysis (Holli et al., 2010a&b), to semi-automated brain region extraction based template (SABRE) analysis (Fujiwara et al., 2008; Levine et al., 2008), to the use of FreeSurfer for volumetric analysis of multiple brain regions (e.g., Strangman et al., 2010; Warner et al., 2010a&b; Yurgelun-Todd et al., 2011).
With all the differences among the studies, the most important take home message is that MRI can be used to detect brain abnormalities in patients with TBI. It is also not surprising that the injuries that are most apparent are observed in more moderate and severe cases of TBI. Further, the volume of lesions can be detected, although whether or not these lesions are in frontal or non-frontal regions does not seem to differentiate between groups on measures of cognitive function (Anderson et al., 1995). Mild TBI patients, nonetheless, evince MR lesions in 30% of a sample of 20 patients (Cohen et al., 2007), and in one study, functional outcome was correlated with lesion volume and cerebral atrophy, although this study did not analyze, separately, mild, moderate, and severe cases of TBI (Ding et al., 2008).
Overall brain volume reduction (atrophy) also seems to be a common finding in what are likely to be more severe patients (e.g., Cohen et al., 2007; Ding et al., 2008; Gale et al., 1995; Gale et al., 2005; Levine et a., 2008; Mackenzie et al., 2002; Trivedi et al., 2007; Warner et al., 2010a; Yount et al., 2002), and there are also volume reductions noted in overall gray matter (e.g., Cohen et al., 2007; Ding et al. 2008; Fujiwara et al., 2008; Schonberger et al., 2009; Trivedi et al., 2007), with a finding also of gray matter volume reduction in the frontal lobe (e.g., Fujiwara et al., 2008; Strangman et al., 2010; Yurgelun-Todd et al., 2011), and in frontal and temporal lobes in some cases (e.g., Bergerson et al., 2004; Gale et al., 2005; Levine et al., 2008). Additionally, Bergerson et al. (2004) reported a correlation between frontal and temporal lobe atrophy and deficits in memory and executive function in patients with a range of severity from mild, to severe (GCS; 3–14).
Overall reduction in white matter has also been reported (e.g., Ding et al., 2008; Levine et al., 2008; Schonberger et al., 2009), as well as white matter reduction at the level of the mesencephalon, corona radiata, centrum semiovale (Holli et al., 2010a&b), and corpus callosum (Holli et al., 2010a&b; Warner et al., 2010a; Yount et al., 2002). Ding and coworkers noted that the changes in white and gray matter over time were correlated with acute diffuse axonal injuries and the latter predicted post-injury cerebral atrophy.
More specific reductions in volume in brain regions have also been observed, including in the hippocampus (Bigler et al., 1997; Himanen et al., 2005; Strangman et al., 2010; Tate et al., 2000; Warner et al., 2010a), amygdala (e.g., Warner et al., 20b10a), fornix (Gale et al., 1995; Tate et al., 2000), thalamus (e.g., Strangman et al., 2010; Warner et al., 2010a; Yount et al., 2002), regions involving the cingulate gyrus (e.g., Gale et al., 2005; Levine et al., 2008; Strangman et al., 2010; Yount et al., 2002), as well as increased lateral ventricles, temporal horns of the lateral ventricles, and/or ventricular brain ratio (e.g., Anderson et al., 1995; Bigler et al., 1997; Gale et al., 1995; Himanen et al., 2005; Wilde et al., 2006; Yount et al., 2002). Reduced volume in subcortical gray matter regions has also been reported (Gale et al., 2005), as has reduced volume in the putamen, precuneus, post-central gyrus, paracentral lobule, parietal cortex, pericalcarine cortex, and supramarginal gyrus (Warner et al., 2010a).
Taken together, these findings suggest that morphometric brain abnormalities are observed in patients with TBI, although many studies did not separate mTBI from moderate and severe TBI. Moreover, in addition to combining mild TBI with moderate and severe cases, the differences among the studies reviewed make the interpretations of findings difficult, and have led to a sponsored work group meeting in 2009, entitled “the Common Data Elements Neuroimaging Working Group.” This work group was established to make recommendations for “common data elements” that will likely be useful for characterizing “radiological features and definitions,” which are critically needed to characterize TBI (Duhaime et al., 2010). This work group was sponsored by multiple national healthcare agencies, including the Defense Centers of Excellence (DCOE), The National Institute of Neurological Diseases and Stroke (NINDS), The National Institute on Disability and Rehabilitation Research (NIDRR), and the Veterans Administration (VA). This work group was also charged with making recommendations for radiological image acquisition parameters that should be standardized in the quest for delineating brain injuries in TBI, particularly given that different imaging acquisition parameters have been used for different applications, as well as for different research studies. Further, if radiological imaging is to be used as surrogate endpoints for evaluating treatment in clinical trials, then some type of standardization of the image acquisition parameters is an important consideration (Duhaime et al., 2010; Haacke et al., 2010).
Haacke et al. (2010) also notes that brain imaging, particularly using more advanced imaging techniques, affords an important and unique opportunity to visualize and to quantify brain injuries in TBI, which is particularly useful in what he describes as the 90% of cases that are categorized as mild. He and his coworkers note that a systematic characterization of brain injuries in TBI will likely lead to increased predictive power in the area of clinical trials and clinical interventions. The new methods that Haacke and coworkers describe (2010) include, DTI, SWI, MRS, SPECT, PET, Magnetoencephalography, and Transcranial Doppler. Haacke et al. (2010) also discuss the importance of combining techniques in the same subjects, such as PET and fMRI.
Selecting optimal protocols has been the focus of investigation in other disorders such as Alzheimer’s disease (e.g., Leung et al., 2009) and schizophrenia (e.g., Zou et al., 2005). One has to keep in mind, however, that for multi-center studies, not all centers have the most up to date, state-of-the-art imaging, and for this reason some compromises need to be made to acquire the best imaging data possible across centers, with a focus on more state-of-the-art and experimental protocols being more possible at research centers. Nonetheless, the points raised by this working group (Duhaime et al., 2010; Haacke et al., 2010) are important and there is much room for improvement in the kind of imaging data and analyses performed in the investigation of TBI. For mTBI this becomes even more crucial as subtle, small changes are unlikely to be detected using more gross radiological measures of brain pathology. Below, we review findings from diffusion imaging studies of mTBI, an important technology for characterizing diffuse axonal and focal axonal injuries, and which is among the most promising imaging tools for revealing subtle, small areas of brain injury in mTBI.
Review of DTI Findings in mTBI
DTI is a sensitive measure of axonal injury that is particularly important for evaluating small and subtle brain alterations that are characteristic of most mTBI. DTI will also likely become an important diagnostic tool for individual cases of mTBI, particularly where MR and CT are negative. With respect to the latter, DTI can depict multifocal and diffuse axonal injuries in individual cases of mTBI. Normative atlases of DTI derived measures that depict anatomical variation in healthy controls can also be created so that individual cases may then be compared with an atlas in order to discern the pattern of pathology in an individual case (e.g., Bouix et al., 2011; Pasternak et al., 2010). We will return to the use of atlases in the section on future directions of research, which follows.
Below we review DTI findings in mTBI. Table 3 lists those studies that focus on mTBI only, or that include other categories such as moderate and severe TBI, but nonetheless conduct statistical analyses separately for the mTBI group. Table 4, on the other hand, includes those studies that do not separate findings in mild TBI from moderate and severe TBI, making findings from these studies more similar to many of the findings reported for morphometry measures in Table 2, where mild, moderate, and severe TBI were often not analyzed separately.
Table 3.
DTI Studies in Mild TBI
| First Author | Year | Type of Study (time post-injury) | Magnet | Subjects (N, gender, age) | DTI Analysis Method and Dependent Measures | Brain Region(s) | Main Finding(s) |
|---|---|---|---|---|---|---|---|
| Arfanakis | 2002 | Acute. (24hrs). In 2 patients, baseline compared with DTI at 1 month follow-up. |
1.5T |
Patients: 5 patients (3M, 2F; mean age 35.6) [GCS 13–15]. Conventional CT normal. Controls: 10 controls (5M, 5F; mean age 28.9 years). |
Analysis and Methods: ROI Analysis. Dependent Measures: Trace, FA and LI. (Lattice Index). |
5 ROI structures with selected volumes (i.e., representative only), in the left and right hemispheres: Anterior and posterior CC, external capsule, anterior and posterior internal capsule. |
|
| Inglese | 2005 | Acute and Chronic. In 20 of the patients, imaging was performed at a mean of 4.05 days post- injury. In the remaining 26, imaging was performed a mean of 5.7 years post- injury. |
1.5T |
Patients: 46 patients (29M, 17F; mean age 36) [GCS 13–15]. Conventional MRI showed contusions in 5 patients and hematomas in 3. Controls: 29 age- and sex-matched controls (15 M, 14 F; mean age 35). Negative findings on conventional MRI. |
Analysis and Methods: Whole-Brain Histogram Analysis and ROI Analysis. Dependent Measures: FA and MD. |
3 ROI structures with selected voxels (i.e., representative, only): the centrum semiovale, CC, and internal capsule. |
|
| Bazarian | 2007 | Acute. 72 hours: post- concussive (PCS) and neurobehavioral testing. At 1 month: quality of life and PCS assessments (n=11). |
3T |
Patients: 6 subjects [GCS 13–15]. Conventional CT normal. Controls: 6 age- and sex- matched orthopedic controls; Conventional CT normal. Subjects (patients and controls) included 8M and 4F; aged 18–31. |
Analysis and Methods: 2 types of whole brain analyses performed: VBM and a novel, quantile analysis. ROI analysis: Regions of interest were also analyzed using a quantile approach. Dependent Measures: FA, Trace. PCS, neurobehavioral battery, and quality of life assessments. |
|
|
| Kraus | 2007 | Chronic. ≥ 6 months out from injury; average 107 months for all TBI subjects. |
3T |
Patients: 37 TBI patients; 20 mTBI (8M, 12F; mean age 35.85) [LOC <30 min], 17 moderate to severe TBI (8M, 9F; mean age 34.88) [LOC >30 min and/or GCS <13]. 5 in each TBI group with other associated trauma. Controls: 18 controls (7M, 11F; mean age 32.83). |
Analysis and Methods: ROI analysis. ROIs drawn on standardized space, FA maps. Dependent Measures: FA, RD, AD, white matter load defined as total number of regions with reduced FA. Neurocognitive measures relevant to attention, memory, and executive function. |
13 ROI structures: anterior and posterior corona radiata, cortico-spinal tracts, cingulum fiber bundles, external capsule, forceps minor and major, genu, body and splenium of the CC, inferior fronto-occipital fasciculus, superior longitudinal fasciculus and sagittal stratum. |
|
| Lipton | 2008 | Chronic. 8 months to 3 years Retrospective study of consecutive admissions of mTBI. |
1.5T |
Patients: 17 mTBI patients (8M, 9F; age 26–70) [GCS 13–15, LOC <20 min]. Negative CT/MRI findings at time of initial injury. Later, at time of scanning, 1 mTBI subject had a small area of signal intensity likely due to gliosis. Controls: 10 controls of similar age and gender distribution. Negative MRI findings at time of scan. |
Analysis and Methods: Whole-brain histogram analysis and Voxel- wise analysis of retrospective cases. Dependent Measures: FA, MD. Histogram parameters, i.e., kurtosis, skew. |
Whole-brain analysis. |
|
| Miles | 2008 | Acute. Average 4 days (range: 1–10). Follow-up at 6 months for neuropsychological measures. |
1.5T |
Patients: 17 patients (11M, 6F; mean age 33.44, range 18–58) [GCS 13–15, LOC <20 min]. Controls: 29 sex- and age-matched controls (15M, 14F; mean age 35, range 18–61). Not matched on education. |
Analysis and Methods: ROI analysis. Dependent Measures: FA, MD. Neuropsychological measures at baseline (≤24 hour of imaging) and 6 month follow-up for 12/17 mTBI. |
Structural ROI using circles: centra semiovale, the genu and the splenium of the CC, and the posterior limb of the internal capsule. |
|
| Niogi | 2008a | Subacute and Chronic. ≥ 1 month (range 1–65 months). ≥ 1 persistent symptom for postconcussive syndrome. |
3T |
Patients: 34 patients (18M, 16F; mean age 37.4, range 16–61). [GCS 13–15]. 11 mTBI- negative MRI findings; 11 mTBI micro- hemorrhage; 12 mTBI-non- specific white matter hyperintensities or chronic hemorrhagic contusions. Controls: 26 controls (19M, 7 7F; mean age 28.3, range 17–58). |
Analysis and Methods: ROI Analysis. Manual ROI, ellipsoid shapes for each ROI. Dependent Measures: FA. Reaction time to Attention Network Task (Fan et al., 2005). |
ROI structures: uncinate fasciculus, anterior corona radiata, anterior aspect of the inferior longitudinal fasciculus, genu of the CC, the cingulum bundle, and the superior longitudinal fasciculus. |
|
| Niogi (Extension of 2008a paper) | 2008b | Subacute and Chronic. ≥1 month post-injury (mean 16.9 months, range 1–53 months). ≥ 1 persistent symptom for postconcussive syndrome. |
3T |
Patients: 43 patients (28M, 15F; mean age 32.4) [GCS 13–15]. Negative findings for conventional MRI images of 12/43 patients. Controls: 23 controls (17 M, 6F; mean age 29.9) Negative findings for conventional MRI. |
Analysis and Methods: ROI analysis. Manual ROI, ellipsoid shapes for each ROI. Dependent Measures: FA. Attention Network Task (Fan et al., 2005), California Verbal Learning Test (CVLT) to test memory performance. |
ROI structures: uncinate fasciculus, anterior corona radiata, anterior aspect of the inferior longitudinal fasciculus, genu of the CC, the cingulum bundle, and the superior longitudinal fasciculus. |
|
| Rutgers | 2008a | Subacute and Chronic. Median 5.5 months (range 0.1–109.3 months). |
1.5T |
Patients: 21 mTBI patients (12M, 9F; mean age 32±9) [GCS 13–15]. 17/21 patients negative findings on MRI; others contusions and 1 extra-axial hematoma. Controls: 11 controls (8M, 3F; mean age 37±9). No known history of positive MRI findings. |
Analysis and Methods: ROI, tractography. Dependent Measures: FA, volume, ADC, number and length of through-passing fibers in each ROI, tractography measure of discontinuity. |
ROI structures: cerebral lobar white matter, cingulum, CC, anterior and posterior limb of the internal capsules, mesencephalon, brain stem, and cerebellum. Cerebral lobar white matter was subdivided into centrum semiovale, frontal lobe, parietal lobe, temporal lobe, and occipital lobe. |
|
| Rutgers | 2008b | Chronic. Average 2.8 months. |
1.5T |
Patients: 39 TBI patients (27M, 12F; mean age 34±12); 24 mild [GCS 13–15]; 9, moderate [GCS 9–12]; and 6, severe TBI [GCS ≤8]. Controls: 10 controls (7M, 3F; mean age 37±9). No known history of positive MRI findings. |
Analysis and Methods: ROI analysis done by drawing ROI manually on the parts of the CC on FA maps of each subject. Dependent Measures: FA, ADC, number of fibers. |
ROI structures: CC genu, body, and splenium. |
|
| Huang | 2009 | “Post-acute”. | 1.5T |
Patients: 10 patients mild TBI (mean age 25.0±11.5; mean education 12.7±4.7 years) [GCS 13–15]. 7 of 10 negative findings on conventional CT/MRI. Controls: 14 age-matched healthy subjects (mean age 27.4±15.2, mean education 12.9±3.2 years). |
Analysis and Methods: Probabilistic tractography, Tract Based Spatial Statistics, MEG Dependent Measures: MEG delta slow waves, FA |
Fronto-occipital fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, uncinate fasciculus, CC, and cingulum bundles. Automated Whole Brain Analysis. |
|
| Lipton | 2009 | Acute. ≤ 2 weeks. |
3T |
Patients: 20 mTBI patients (9M, 11F; mean age 33.4) [CGS 13–15, LOC <20 min, PTA <24 hr]. Controls: 20 matched controls (9M, 11F; mean age 34.2). |
Analysis and Methods: Voxel-wise analysis. Dependent Measures: FA, MD. |
Automated analysis of whole brain. |
|
| Lo | 2009 | Chronic. ≥ 2 years. |
1.5T |
Patients: 10 patients (5M, 5F; age range 20–51) [CGS 13–15]. For those who had conventional MR/CT, the findings were negative. Following research scan, 1 subject had a small focal area showing lobar gliosis. Controls: 10 controls (5M, 5F; mean age 44). Controls were patients referred for MRI due to headache and had no history of head trauma. |
Analysis and Methods: ROI. Dependent Measures: FA, ADC. |
ROIs placed in the genu and splenium of the CC, posterior limb of the internal capsule, and in the pontine tegmentum. |
|
| Geary | 2010 | Chronic. ≥ 6 months. |
3T |
Patients: 40 mTBI patients (17M, 23F; mean age 34.53) [American Congress of Rehabilitation Medicine, 1993 criteria]. 14 subjects had previous head trauma. Negative MRI/CT findings. Controls: 35 controls (16M, 19F; mean age 32.54) matched to patients on age, education, years of employment, and estimated premorbid intelligence. |
Analysis and Methods: ROI. Dependent Measures: FA. |
ROIs structures: anterior and posterior corona radiata, corticospinal tracts (including parts of the corticopontine tract and superior thalamic radiation), external capsule, cingulum, forceps minor, forceps major, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, sagittal stratum, and body, genu, and splenium of CC. |
|
| Holli(b) | 2010 | Acute. ≤ 3 weeks. |
1.5T |
Patients: 42 patients (17 M, 25F; mean age, 38.8) [GCS 13–15]. DTI analyses were performed on 34 patients. Negative CT/MRI findings. Controls: 10 age- and sex- matched controls (4M, 6F; mean age, 39.8). |
Analysis and Methods: Texture analysis (MaZda). Dependent Measures: FA, ADC. |
Regions corresponding to the mesencephalon, centrum semiovale, and CC. |
|
| Maruta | 2010 | Subacute to Chronic. 6 weeks and 5 years (mean 2.7 years). |
3T |
Patients: 17 patients (10M, 7F; age range 20–52), [GCS 15 with chronic postconcussive syndrome, recruited from clinics]. 8 normal, 9 showed abnormalities. Controls: 9 controls (6M, 3F; age range 19–31). No prior history of TBI. |
Analysis and Methods: ROIs selected a priori. Dependent Measures: FA. |
Anterior corona radiata, genu of the CC, uncinate fasciculus, cingulum bundle, forceps major, and superior cerebellar peduncle. |
|
| Mayer | 2010 | Acute. ≤ 21 days (mean 12 days). |
3T |
Patients: 22 patients (mean age 27.45) [GCS 13–15, LOC <30 min, PTA <24hr]. Controls: 21 sex-, age-, and education-matched controls (mean age 26.81). |
Analysis and Methods: ROI. Dependent Measures: FA, AD, RD. |
Genu, splenium, and body of the CC, as well as the superior longitudinal fasciculus, the corona radiata, the superior corona radiata, the uncinate fasciculus, and the internal capsule for both hemispheres. |
|
| Zhang | 2010 | Subacute. 30 (±2) days. |
3T |
Patients: 15 student-athletes with mTBI (mean age 20.8 years) [Grade 1 MTBI according to Cantu 2006. GCS not reported]. Negative MRI findings. Controls: 15 normal student- athletes with no history of mTBI (mean age 21.3). Negative MRI findings. The entire sample was 70%M and 30%F. |
Analysis and Methods: Voxel-wise whole brain analysis and ROI. Dependent Measures: FA, ADC. |
The CC was chosen as a primary ROI and subdivided into the genu, body, and splenium. In addition, the right hippocampus, left and right dorsolateral prefrontal cortexes were evaluated. |
|
| Warner | 2010b | Acute. ≤1 week Follow-up at 4–16 months. |
3T | Patients: 24 patients with diffuse traumatic axonal injury (16M, 8F; mean age 27.2) [mean GCS 6.4; range not reported]. Controls: None. |
Analysis and Methods: Automated Segmentation and Volumetric Analysis (FreeSurfer). Dependent Measures: Regional Volumes, FA, MD. |
CC, fornix body, bilateral fornix crus, bilateral perforant pathway, cingulum, uncinate fasciculus, and inferior fronto-occipital fasciculus. |
|
| Bazarian | 2011 | Acute. (72 hours post-injury). Prospective cohort study. Subjects underwent DTI pre- and postseason within a 3-month interval. (Concussed subjects underwent repeat testing ≤ 72 hours of injury. Subjects who did not suffer a concussion during the season underwent repeat testing ≤ 1 week of the end of the sports season 3 months after initial testing). |
3T |
Patients: One athlete concussion [witnessed LOC >20 min, transient amnesia or confusion; GCS not reported] and 8 athletes with 26–399 subconcussive head blows. Controls: 6 control subjects. 2 control subjects had isolated minor orthopedic injuries. |
Analysis and Methods: Whole brain analysis. FA and MD changes were measured in five ROIs. Dependent Measures: FA, MD. |
External capsule, posterior and anterior CC, posterior and anterior limb of the internal capsule. |
|
| Cubon | 2011 | Subacute and Chronic. ≥1 month (mean 115 days, SD 104 days) for the sports-related concussion subjects. ≥ 1 year for subjects with moderate and severe TBI. |
3T |
Patients: 10 college students with concussion (5M, 5F; mean age 19.7 years) [Diagnosis based on International Consensus Agreements 2000, GCS not reported]. 2 moderate TBI subjects (mean age 20) [GCS 9–12]. 3 severe TBI (mean age 47.3) [GCS ≤8]. Controls: 10 sex- and age- matched athletes (5M, 5F; mean age 20.4 years). Sex- and age-matched controls, for the 2 moderate (mean age 22) and 3 severe TBI subjects (mean age 46). |
Analysis and Methods: TBSS. Dependent Measures: FA, MD. |
Whole brain WM skeleton (TBSS). |
|
| Davenport | 2011 | Chronic. 2–5 years after blast injury. |
3T |
Patients: 25 veterans with mTBI (24M, 1F; mean age 36) [LOC <30 min, PTA <24 hours, and neurological symptoms; GCS not reported). Controls: 33 veterans (28 M, 5F; mean age 32.5). |
Analysis and Methods: 20 standard probabilistic tractography-based ROIs. Dependent Measures: FA. |
Forceps major, forceps minor, anterior thalamic radiations, hippocampal portion of cingulum, cingulum, corticospinal tract, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, temporal portion of superior longitudinal fasciculus, uncinate. |
|
| Grossman | 2011 | Chronic. MRI and a neuropsychological battery either ≤ 1 year after injury, or >1 year after injury. |
3T |
Patients: 22 patients (14M, 8F; mean age 38.2) [GCS 13–15]. Controls: 14 healthy controls (9M, 5F; mean age 36.5) matched to patients according to gender, age, and formal education |
Analysis and Methods: ROI. Dependent Measures: MK, FA, MD. |
Thalamus and the anterior limb, genu, and posterior limb of the internal, the splenium of the CC, and the centrum semiovale. |
|
| Henry | 2011 | Acute. 1–6 days post-concussion. For patients, follow-up 6 months later; for controls, 2nd scan 18 months after initial scan. |
3T |
Patients: 18 athletes with mTBI (all M; mean age 22.08) [Diagnosis based on American Academy of Neurology Quality Standards Subcommittee and Neurology, 1997; GCS not reported]. Controls: 10 athletes (all M; mean age 22.81). |
Analysis and Methods: Voxel-based approach (VBA). Dependent Measures: FA, MD, AD. |
A voxel-wise 2 × 2 repeated-measures analysis of variance (ANOVA) using SPM8 to the derived scalar images (FA, AD, and MD) was used. |
|
| Lange | 2011 | Subacute. 6–8 weeks. |
3T |
Patients: 60 mTBI patients (43M, 17F; mean age 30.8) [Closed head injury and; LOC >1minute, PTA >15 minutes, GCS <15, or abnormality on CT]. n=41 negative CT findings, N=15 positive CT findings, and n=4 not ordered. N=21 with postconcussive disorder based on ICD-10; N=39 without postconcussive disorder. Controls 34 controls (25M, 9F; mean age 37.1) with orthopedic/ soft- tissue injuries. N=0 positive findings, n=3 negative findings, and n=31 not ordered. 52.9% of controls met ICD-10 criteria for postconcussive disorder. |
Analysis and Methods: ROIs. Dependent Measures: FA, MD. |
Genu, body, and splenium of the CC. |
|
| MacDonald | 2011 | Subacute. ≤ 90 days. |
1.5T |
Patients: 63 patients (all M, median age 24) [Diagnosis based on U.S. military clinical criteria for traumatic brain injury; GCS not reported]. Controls: 21 controls (all M, median age 31) exposed to blasts, but none with sustained TBI. |
Analysis and Methods: ROI. Dependent Measures: Relative anisotropy (RA), MD, AD, RD. |
Genu and splenium of the CC, the right and left middle cerebellar peduncles, the right and left cerebral peduncles, the right and left uncinate fasciculi, and the right and left cingulum bundles. |
|
| Matthews | 2011 | Self-reported history of concussion. | 3T |
Patients: 22 patients with a history of blast- related TBI. [LOC <30min, PTA <25 hr, CGS 13–15]. 11 TBI patients with Major Depressive Disorder (MDD) (all M, mean age 26.8). 11 TBI patients without MDD (all M, mean age 30.3). Controls: None. |
Analysis and Methods: VBA. Dependent Measures: MD, 3 Lambda values, 9 eigenvalues λ1, λ2, λ3, total MD, FA. |
|
|
| Matsushita | 2011 | Acute. Median 3.5 days. |
1.5T |
Patients: 20 patients (18M, 2F; mean age 39.3): 9 mild TBI [GCS 13–15, LOC <30 min, PTA <24 hr]. 11 moderate TBI [LOC ≥30 min and/or GCS 9–12]. Controls: 27 matched controls (13M, 14F; mean age 42.9). |
Analysis and Methods: ROI. Dependent Measures: FA. |
Genu, stem, and splenium of the CC and the corona radiata, anterior limb of the internal capsule, posterior limb of the internal capsule, frontal white matter, and occipital white matter of the periventricular white matter. |
|
| Messe | 2011 | Acute. 7–28 days (subacute phase), Subacute. 3–4 months (late phase). |
1.5T |
Patients: 23 patients [LOC <30 min, CGS 13–15, PTA <24 hr, or neurological symptoms]; 11 with good outcome (8M, 3F; mean age 27.8), 12 with poor outcome (7M, 5F; mean age 31.3). 7 of 11 with good outcome had negative MRI findings and 6 out of 12 poor outcome had negative MRI findings. Controls 23 controls (12M, 11F; mean age 30). |
Analysis and Methods: VBM, TBSS. Dependent Measures: FA, MD, AD, RD. |
Whole Brain. |
|
| Smits | 2011 | Subacute. Average 30.6 days. |
3T |
Patients: 19 patients included (10M, 9F; mean age 26) [GCS 13–15]. Controls: 12 controls (8M, 4F; mean age 28). |
Analysis and Methods: TBSS. Dependent Measures: FA, MD. |
Whole Brain. |
|
| Sponheim | 2011 | Chronic. Several Months. |
1.5T |
Patients: 9 patients (all M; mean age 33.7) [Diagnosis based on American Congress of Rehabilitation Medicine Special Interest Group on Mild Traumatic Brain Injury and the concussion grading system by the American Academy of Neurology; GCS not reported]. Controls: 8 controls (all M; mean age 30.3). |
Analysis and Methods: ROI. Dependent Measures: Electroencephalogram (EEG) phase synchronization and FA. |
Forceps major, forceps major and anterior thalamic radiations. |
|
| McAllister | 2012 | Acute. Preseason and ≤ 10 days of a diagnosed concussion. |
3T | Subjects: 10 athletes (all M; age range 15–23) with mTBI [Diagnosis by certified athletic trainer or team physician; GCS not reported]. Controls: None. |
Analysis and Methods: Strain and strain rate calculations based on Dartmouth Subject- Specific FE Head model (helmets worn to record head impacts during play on the field). DTI maps for CC. Dependent Measures: FA, MD, Strain, Strain Rate. |
CC. |
|
Key: M=Male; F=Female; PTA=Posttraumatic Amnesia; LOC=Loss of Consciousness; GCS=Glascow Coma Scale; CC=Corpus Callosum; ADC=Apparent Diffusion Coefficient; FA=Fractional Anisotropy; MD=Mean Diffusivity; RD=Radial Diffusivity; AD=Axial Diffusivity; DKI=Diffusional Kurtosis Imaging; DTI=Diffusion Tensor Imaging; MK=Mean Kurtosis; ROI=Region of Interest; TBSS=Tract-Based Spatial Statistics.
This table presents findings derived from diffusion tensor imaging (DTI) including: Trace, Mean Diffusivity (MD), Fractional Anisotropy (FA), Axial Diffusivity (AD), Radial Diffusivity (RD), and Mean Kurtosis (MK). In studies where non-DTI diffusion weighted imaging (DWI) was also presented we include the main measure, Apparent Diffusion Coefficient (ADC). In the description of subjects, we provide, when available, the Glasgow Coma Scale (GCS) score, Loss of Consciousness (LOC) duration, and Posttraumatic Amnesia (PTA) duration. Acute is defined as under 1 month, subacute is defined as >1 month but less than 6 months, and chronic is defined as >6 months.
Table 4.
DTI Studies with Different DTI Severities Including Mild
| First Author | Year | Type of Study [time post-injury] | Magnet | Subjects (N, gender, age) | DTI Analysis Method and Dependent Measures | Brain Region(s) | Main Finding(s) |
|---|---|---|---|---|---|---|---|
| Huisman | 2004 | Acute <7 days. |
1.5T |
Patients: 20 patients (15M, 5F; mean age 31). [GCS 4–15]. Controls: 15 healthy subjects with a matching age distribution (mean age 35). |
Analysis and Methods: ROI Analysis. Dependent Measures: FA, ADC. |
Posterior limb of the internal capsule (bilaterally) and the splenium of the CC. Measurements in the thalamus and putamen served as internal references. |
|
| Salmond | 2006 | Chronic > 6 months. |
3T |
Patients: 16 patients (13M, 3F; mean age 32) [Median GCS 7, range 3–13]. Controls: 16 controls [12M, 3F; mean age 34]. |
Analysis and Methods: Voxel based analysis. Dependent Measures: FA, MD. |
Maps of FA and MD were calculated from the diffusion-weighted images. |
|
| Benson | 2007 | Acute to Chronic. Variable from days to years (mean 35.3 months, range 3 days to 15 years). |
1.5T |
Patients: 20 patients (13M, 7F; mean age 35.5) [GCS 3–15]. 6 mTBI and 14 moderate/severe TBI All CT+ except N=3 mTBI. Controls: 14 age-matched controls (mean age 27.5). |
Analysis and Methods: White matter whole-brain FA histogram parameters. Dependent Measures: FA, Kurtosis, Skewness. |
Whole-brain analysis using histogram shape parameters. |
|
| Kumar | 2009 | Acute. 5–14 days. |
1.5T |
Patients: 83 patients [62M, 21F; mean age 34.25]. 26 mTBI [GCS 13–15, mean 14.5]. 57 moderate TBI [GCS 9–12]. All were positive for clinical findings on CT at injury. Controls: 33 age- and sex-matched controls (22M, 11F; mean age 32.1). |
Analysis and Methods: ROI. Dependent Measures: FA, RD, MD, AD. |
CC (divided into 7 segments). |
|
| Levin | 2010 | Chronic. Post-injury interval 871.5 days. |
3T |
Patients: 37 veterans (mean age 31.5) with mild or moderate TBI [GCS not provided]. 5 had positive findings on clinical MRI. Controls: 15 veterans without a history of TBI or exposure to blast (mean age 31.4), including 7 subjects with extracranial injury (mean post-injury interval 919.5 days), and 8 who were uninjured. |
Analysis and Methods: Tractography, standard single-slice ROI measurement, and voxel-based analysis. Manually-traced ROIs on a slice-by-slice basis, and quantitative fiber tractography to assess white matter integrity Dependent Measures: FA, ADC |
Non-tractography ROI protocols included the total CC (including genu, body, splenium, and total), and the right and left anterior and posterior limbs of the internal capsule. |
|
| Hartikainen | 2010 | Acute. 3 weeks. |
1.5T |
Patients: 18 patients (6M, 12F; 11 asymptomatic and 7 symptomatic; mean age 43) with mild and moderate TBI. All moderate subjects had positive findings on clinical CT or MRI. Controls: None. |
Analysis and Methods: ROI Dependent Measures: FA, ADC |
Thalamus, internal capsule, centrum semiovale, and mesencephalon. |
|
| Little | 2010 | Chronic. ≥12 months. |
3T |
Patients: 12 patients with mTBI (mean age 31.2). 12 moderate to severe TBI (mean age 33.3) Severity was based on duration of Loss of Consciousness and Post-Traumatic Amnesia. 4 mTBI and 9 moderate to severe patients had positive findings on MRI or CT. Controls: 12 age- and education-matched controls (mean age 30.8). |
Analysis and Methods: ROI. Dependent Measures: FA. |
12 ROIs in cortical and CC structures and 7 subcortical ROIs (anterior, ventral anterior, ventral lateral, dorsomedial, ventral posterior lateral, ventral posterior medial, and pulvinar thalamic nuclei). |
|
| Singh | 2010 | Acute to Chronic. Mean ~1 month. |
1.5T |
Patients: 12 patients (mean age 28) with mild to moderate brain injuries (loss of consciousness for <1 h) primarily due to falls, assault or traffic accidents. Controls: 10 age-matched volunteers (mean age 27). No evidence of neurological disease on MR imaging. |
Analysis and Methods: ROI analysis. Dependent Measures: FA, MD, AD, RD. |
Differences in the FA maps between each TBI subject and the control group were computed in a common space using a t-test, transformed back to the individual TBI subject’s head space, and then thresholded to create ROIs that were used to sort tracts from the control group and the individual TBI subject. Tract counts for a given ROI in each TBI subject were compared to group mean for the same ROI in order to quantify the impact of injury along affected pathways. The same procedure was used to compare the TBI group to the control group in a common space. |
|
| Brandstack | 2011 | Acute. Mean 7 days |
1.5T |
Patients: 22 patients (GCS 6–15) admitted to the emergency department for TBI. On clinical MRI 7 were negative for findings, 15 showed cortical contusions or traumatic axonal injury. Controls: 14 healthy subjects. |
Analysis and Methods: ROI analysis. Dependent Measures: ADC. |
Measurements were performed at 46 different locations including bilateral cortical, subcortical and deep brain structures. |
|
| Ljungqvist | 2011 | Acute. Within 11 days and at 6 months post-injury. |
1.5T |
Patients: 8 patients with TBI (4M, 4F; mean age 36.4) [GCS 3–14]. All patients were CT negative. Controls: 6 controls (3M, 3F; mean age 33.1). |
Analysis and Methods: ROI analysis. Dependent Measures: FA, Trace, AD, RD. |
Polygonal ROIs were manually placed in the CC. |
|
| Yurgelun-Todd | 2011 | Not reported. | 3T |
Patients: 15M veterans with TBI (mild, moderate and severe according to The Ohio State University - TBI Identification Method). Controls: 17M controls: 10 civilians, 6 veterans. |
Analysis and Methods: ROI analysis. Dependent Measures: FA, MD. |
ROIs for major white matter tracts were created for the genu and the cingulum using the Johns Hopkins University White Matter Labels Atlas. |
|
Key: M=Male; F=Female; GCS=Glasgow Coma Scale; CC=Corpus Callosum; ADC=Apparent Diffusion Coefficient; FA=Fractional Anisotropy; MD=Mean Diffusivity; RD=Radial Diffusivity; AD=Axial Diffusivity; ROI=Region of Interest.
This table presents findings derived from Diffusion Tensor Imaging (DTI), including: Trace, Mean Diffusivity (MD), Fractional Anisotropy (FA), Axial Diffusivity (AD), Radial Diffusivity (RD), and Mean Kurtosis (MK). In studies where non-DTI diffusion weighted imaging (DWI) was also presented, we include the main measure, Apparent Diffusion Coefficient (ADC). In the description of the subjects, we provide, when available, the Glascow Coma Scale (GCS) score, Loss of Consciousness (LOC) duration, and Posttraumatic Amnesia (PTA) duration. Acute is defined as under 1 month, subacute is defined as >1 month but greater than 6 months, and chronic is defined as >6 months.
Arfanakis and coworkers (2002) were the first to use DTI to investigate diffuse axonal injuries in mTBI. They investigated FA and MD in anterior and posterior corpus callosum, external capsule, and anterior and posterior internal capsule in 5 patients with acute mTBI (within 24 hours of injury) and 10 controls. These brain regions were selected as likely showing damage based on post-mortem findings. Results showed no mean diffusivity (MD) differences between mTBI patients and controls. Group differences were, however, observed for the corpus callosum and the internal capsule, where fractional anisotropy (FA) was reduced in the mTBI group compared with controls. Importantly, the latter findings are consistent with histopathology findings in mTBI patients who died from other causes (e.g., Adams et al., 1989; Bigler et al., 2004; Blumbergs et al., 1994; Oppenheimer et al., 1968). These investigators concluded that DTI is an important early indicator of brain injury in mTBI and has the potential for being an important prognostic indicator of later injury. These investigators were also prescient in noting that longitudinal studies are needed to understand the dynamic nature of mTBI and how it may reflect changes in brain alterations over time. They did not, however, include a follow up scan of subjects in their study.
Inglese and coworkers (2005) used DTI measures to investigate both acute (n=20 mTBI mean of 4 days post-injury) and chronic mTBI patients (n=26 mTBI, mean of 5.7 years post-injury) compared with controls (n=29). These investigators used FA and MD measures of diffusion to detect abnormalities in the centrum semiovale, the corpus callosum, and the internal capsule. They reported FA reduction in all three structures in mTBI compared with controls, as well as increased MD in the splenium of the corpus callosum, which was higher in the acute sample, but lower in the posterior limb of the internal capsule compared to the chronic sample. These findings are similar to the Arfanakis et al. (2002) study, although these investigators also included patients with chronic TBl. These findings also highlight the importance of detecting brain alterations that change over the course of brain injury.
Miles et al. (2008) also investigated mTBI patients on average 4 days post-injury and again at 6 months follow up on neuropsychological measures (only). They reported increased MD and reduced FA in the centrum semiovale, genu and splenium of the corpus callosum, and in the posterior limb of the internal capsule in mTBI compared with controls. Moreover, 41% of mTBI cases evinced cognitive impairments at baseline, and 33% at follow up 6 months later. More specifically, while there was no correlation between baseline measures of MD/FA and cognitive measures, baseline MD and FA correlated significantly with cognitive measures at follow up, with FA predicting executive function, and a trend for MD to be associated with reaction time. These findings suggest that there may be predictors of cognitive function from baseline measures of impairment as measured on DTI, even when there are no correlations between these measures and cognitive measures at baseline. These investigators also did not include follow up DTI scans at 6 months, only cognitive follow up.
Other investigators examining acute and subacute time periods report similar findings. For example, one study of mild and moderate TBI was conducted within 5 to 14 days post-injury in patients who had positive findings on clinical CT. TBI patients showed reduced FA in the genu of the corpus callosum (mTBI only) and increased radial diffusivity (RD) in the genu of the corpus callosum in both mild and moderate TBI (Kumar et al. 2009). Matsushita and coworkers (2011) investigated both mild and moderate TBI patients with a median of 3.5 days post-injury. They reported decreased FA in the splenium of the corpus callosum in mTBI compared with controls. Other parts of the corpus showed reduced FA in the moderate group including genu, stem, and splenium. These findings suggest more abnormalities in the moderate TBI group.
Bazarian and coworkers (2007) acquired DTI data from mTBI patients within 72 hours of brain trauma and they included a measure of postconcussive symptoms and quality of life assessments. They repeated these assessments one month later. Decreased trace was reported particularly in the left anterior internal capsule, and increased median FA was reported in the posterior corpus callosum in mTBI compared with controls. Of note, trace measures correlated with postconcussive scores at 72 hours and at 1 month post-injury and FA values correlated with 72 hour postconcussive score as well as with a test of visual motor speed and impulse control. All subjects evinced normal findings at time of injury on conventional CT, suggesting that normal findings on conventional CT and MRI are inadequate for characterizing the kind of injury observed in mTBI.
The findings of lower trace and increased FA, however, are more perplexing as the expectation is that if there is damage to the integrity of white matter then FA will be reduced, and MD likely increased, and this is often reported (see Table 3 and 4). An increase in FA and a decrease in MD may indicate axonal swelling that occurs early in the course of injury and may correlate with poor clinical outcome. Bazarian and coworkers (2007) have also suggested this possibility.
Bazarian and coworkers (2011), in another study that involved one athlete with a concussion and an additional 8 athletes with multiple (26–399) subconcussive blows to the head, reported FA and MD changes that were greatest in the one concussed athlete, intermediate in the subconcussive group, and lowest for controls. Of note, they observed that both FA and MD changed in both directions, i.e., increases and decreases were observed. Kou and coworkers (2009) suggest that increased MD and decreased FA may indicate vasogenic edema, which will likely resolve over time, whereas increased FA and reduced MD may indicate cytotoxic edema, reflected by axonal swelling and more restricted diffusion of water, thereby explaining the increase in FA and decrease in MD. Thus increased FA and associated decreased MD, early in the course of brain injury, may indicate a poor prognosis and hence identifying this group early post-injury may make early interventions possible. Here, measures such as free water, which characterize water as belonging to tissue versus extracellular water (e.g., Pasternak et al., 2009), may be helpful in determining intracellular versus extracellular edema, i.e., cytotoxic versus vasogenic edema. Such measures are just beginning to be used in mTBI by our group (e.g., Pasternak et al., 2011a&b).
A further issue is whether or not an observed increase in FA early in the course of injury is predictive of poorer outcome, or if an increase in FA at any time over the course of the injury is a poor indicator of outcome. This question is relevant, particularly given that some investigators do not report increased FA at 24 hours post-injury (e.g., Arfanakis et al., 2002), while others report increased FA at 72 hours (Bazarian et al., 2007). Of interest here, Henry et al. (2011) scanned 18 athletes with mTBI at 1 to 6 days post-injury, later in post- injury than either the Arfanakis or Bazarian studies, and they also reported increased FA and decreased MD in dorsal corticospinal tracts and in the corpus callosum at both baseline and at 6 month follow up. Further, Mayer et al. (2010) scanned mTBI patients 21 days post-injury, on average 12 days post-injury. They, too, reported increased FA and reduced radial diffusivity (RD) in the corpus callosum in mTBI patients compared with controls. Finally, a study by Hartikainen et al. (2010) compared 11 asymptomatic TBI patients with 7 symptomatic TBI patients, which included both mild and moderate cases, with moderate TBI patients all having abnormal findings on clinical CT or MRI. In comparing these two groups, the symptomatic patients showed increased FA and lower ADC in the mesencephalon compared with the asymptomatic patients. Thalamus, internal capsule, and centrum semiovale did not differentiate between these two groups. There was no control group. Nonetheless, the notion that more symptomatic patients showed increased FA is important and could serve as a biomarker for predicting those patients who have, or will have, a poorer outcome.
The implications of increased FA and reduced MD, and whether these measures are indicators of poor outcome clearly needs further investigation. A longitudinal study would help to follow the natural progression of brain injury in mTBI over time in order to determine the significance of increased versus decreased FA that is observed in mTBI.
Other noteworthy findings of brain injury in mTBI include reduced FA in frontal white matter, including dorsolateral prefrontal cortex, where Lipton et al. (2009) reported several clusters of increased MD, which were correlated with worse executive functioning in a group of acute mTBI patients compared with controls. Other findings include abnormalities in mesencephalon, centrum semiovale, and corpus callosum (Holli et al. 2010b), with findings for the mesencephalon being correlated with verbal memory in mTBI patients. Additionally, reduced FA has been reported in the anterior corona radiata, in the genu of the corpus callosum, and in the left superior cerebellar peduncle (Maruta et al., 2010). In the latter study, gaze positional errors in an eye movement task were correlated with mean FA values of the right anterior corona radiata, the left superior cerebellar peduncle, and the genu of the corpus callosum, all tracts that support spatial processing and are involved in attention, leading these investigators to suggest that gaze errors might be a useful screening tool for mTBI.
Kraus and coworkers (2007) focused on chronic patients who had mild (n=20), moderate and severe (n=17) injury, and 18 controls. They investigated FA as well as radial diffusivity (RD), axial diffusivity (AD), and white matter load; the latter defined as the total number of regions with reduced FA. She and her coworkers reported FA decreases in all thirteen brain regions examined in the moderate to severe group. In the mTBI group, FA was reduced in only the corticospinal tract, sagittal striatum, and superior longitudinal fasciculus. Both AD and RD were increased in several white matter regions in the moderate to severe group, whereas in the mTBI group only increases in AD were observed, suggesting that myelin damage is not present in mTBI but is present in moderate to severe TBI. These investigators concluded that white matter changes that indicate diffuse axonal injury in TBI show alterations along a spectrum from mild to severe TBI.
Lipton et al. (2008) also investigated more chronic patients. They compared 17 mTBI patients who had negative CT/MRI findings at the time of injury, with 10 controls who also showed negative findings on MRI. One of the mTBI subjects subsequently was observed to develop a small area of increased signal intensity, likely the result of gliosis. Decreased FA and increased MD were observed in the corpus callosum, subcortical white matter, and in the internal capsules, bilaterally. These brain regions are similar to those observed as abnormal in the acute studies noted above (i.e., Arfanakis et al., 2001; Inglese et al., 2005; Bazarian et al., 2007; Miles et al., 2008).
Niogi and colleagues (2008a) investigated a group of patients 1 to 65 months following injury; all characterized as having more than one symptom of postconcussive syndrome. Subjects included 34 mTBI patients that were separated into those with negative MRI findings (n=11), those with micro-hemorrhage (n=11), and those with white matter hyperintensities or chronic hemorrhagic contusions, compared with controls (n=26). They investigated six regions of interest and reported reduced FA in the anterior corona radiata (41%), the uncinate fasciculus (29%), the genu of the corpus callosum (21%), the inferior longitudinal fasciculus (21%), and the cingulum bundle (18%). Reaction time was correlated with a number of damaged white matter structures and it was noted that 10 of the 11 of the mTBI patients with negative MRI findings showed reductions in FA relative to controls. In a slightly larger sample of mTBI patients, this group also reported significant correlations between attentional control and FA reduction in the left anterior corona radiata, as well as significant correlations between memory performance and reduced FA in the uncinate fasciculus, bilaterally (Niogi et al., 2008b). These cognitive correlates with white matter fiber tract abnormalities findings are consistent with what would be expected given the function of these tracts, further suggesting that FA may be useful as a biomarker for neurocognitive function and dysfunction in mTBI.
Given the different magnet strengths, with some conducted on a 1.5T magnet, and others conducted on a 3T magnet (see Tables 3 and 4), as well as differences in the analysis methods employed, and the dependent measures used, as well as differences in the selection of brain regions to investigate, in addition to differences in the post-injury time of the study, and differences in whether subjects had positive or negative findings on conventional CT or MRI, it is surprising that there is as much convergence and consistency with respect to the detection of brain abnormalities in mTBI using DTI. Given the DTI findings reviewed above, and listed in Tables 3 and 4, as well as the frequently of negative CT and structural MRI scans, it is also quite clear that DTI is by far the most sensitive in vivo method to detect subtle brain abnormalities in mTBI.
While each of the 43 DTI studies investigating mTBI, and included in Tables 3 (n=32) and 4 (n=11), report some DTI abnormalities, their anatomical location does not always converge. This lack of convergence is not, however, surprising, given the heterogeneity of brain injuries, as well as the variability in these studies between time of injury and DTI scan. Some regions, however, are reported more often than the others, which might further suggest their increased vulnerability to axonal injury.
For example, in reviewing specific brain regions, some, as noted above, including the corpus callosum and parts of the corpus, are abnormal in mTBI (e.g., Arfanakis et al., 2001; Bazarian et al., 2007; Grossman et al., 2011; Henry et al., 2011; Holli et al., 2010(b); Huisman et al., 2004; Inglese et al., 2005; Kumar et al., 2009; Lipton et al., 2008; Little et al., 2010; Ljungqvist et al., 2011; Lo et al., 2009; Messe et al., 2011; Maruta et al., 2010; Matthews et al., 2011; Mayer et al., 2010; McAllister et al., 2012; Miles et al., 2008; Niogi et al., 2008a&b; Rutgers et al., 2008a&b; Singh et al., 2010; Smits et al., 2011; Warner et al., 2010(b); Yurgelun-Todd et al., 2011).
Rutgers and coworkers (2008a) have also reported reduced FA predominantly in 9 major regions in mTBI patients, and one of these regions included the corpus callosum. In a separate study by this group of investigators (Rutgers et al., 2008b), mTBI and moderate TBI subjects were included. Patients with mTBI or less than 3 months post-injury showed reduced FA and increased ADC in the genu of the corpus callosum, whereas patients with greater than 3 months post-injury showed no such differences. This group of investigators also showed that more severe trauma was associated with FA reduction in the genu and splenium of the corpus callosum, along with increased ADC and fewer numbers of fibers. These findings suggest that there may be a reversal of damage to the corpus callosum in patients with mTBI who recover after 3 months, and that more severe damage to the corpus callosum may be associated with a worse outcome. Again, a longitudinal study that investigates the course of injury over time would provide important information regarding the staging, progression, and possible recovery and reversal of brain injuries over time.
Huisman et al. (2004) included TBI patients with Glasgow Coma Scale scores between 4 and 15, and hence moderate and severe were not separated from mild in evaluating the corpus callosum. Matthews et al. (2011) examined mTBI patients with major depressive disorder and those without a major depressive disorder. These investigators found that those with a major depressive disorder showed reduced FA in the corpus callosum (also corona radiata and superior longitudinal fasciculus) compared with those without a major depression. Zhang et al. (2010), on the other hand, reported no differences in the corpus callosum using either whole brain analysis or region of interest analyses between mTBI athletes and controls, but mTBI patients did show greater variability of FA in the genu and in the body of the corpus callosum than did controls. Lange et al. (2011) also reported no differences in FA or MD between mTBI and controls, although they did report a non-significant trend for an increase in MD in the splenium of the corpus callosum in mTBI compared with controls. MacDonald et al. (2011) also reported no differences in genu or splenium of corpus callosum between mTBI and controls. The controls in this study, however, were 21 controls with blast exposure but without head trauma. It may be, however, that exposure to blasts results in subconcussive injury to the brain as is reported in sports-related injuries (e.g., Cubon et al., 2011; McAllister et al., 2012). Accordingly, fewer findings between groups might be accounted for by the fact that the control group is more similar to the mTBI group. Levin et al. (2010) also did not find differences for the corpus callosum between TBI patient and controls on FA or ADC measures, but here mTBI cases were not separated from moderate TBI and the controls were 15 veterans, 7 with extracranial injury.
Again, the issue of using a military population as a control group needs more careful consideration as many of these individuals may have sustained blast injuries where they did not experience loss of consciousness but which may, nevertheless, have resulted in subtle alterations to the brain (and thus use of such controls might result in a decrease in the sensitivity of the imaging methods).
Davenport and coworkers (2011) selected veterans with and without blast exposure in their study where they investigated 25 veterans returning from Operation Enduring Freedom and Operation Iraqi Freedom who had been exposed to blasts and subsequently developed symptoms indicative of mTBI, and they included a comparison group comprised of 33 veterans who had not experienced either blast exposure or head injury during their tour of duty. These investigators reported diffuse and global patterns of reduced FA in white matter in the blast exposure group compared to the group not exposed to blast. Furthermore, those who experienced more than one blast related mTBI showed a larger number of reduced FA voxels in the brain. Interestingly, and surprisingly, 58% of the no blast group had experienced a previous mTBI as civilians, prior to entering the service. This group did show differences, however, compared with the blast exposure group with symptoms. Here the issue may not be exposure per se, but the differentiation between those with and without symptoms being more important in differentiating between groups. Yurgelun-Todd et al. (2011) also investigated a sample of 15 veterans with mild, moderate, and severe TBI and compared them with 10 civilians and 6 veterans. They reported decreased FA in left cingulum and genu of the corpus callosum. The TBI group showed greater impulsivity. In addition, both total and right cingulum FA reduction was correlated with increased suicidal ideation and increased impulsivity.
Other brain regions reported as abnormal in mTBI include the internal capsule (Arfanakis et al., 2002; Bazarian et al., 2007; Bazarian et al., 2011; Cubon et al., 2011; Grossman et al., 2011; Huisman et al., 2004; Inglese et al., 2005; Lipton et al., 2008; Lo et al., 2009; Mayer et al., 2010; Miles et al., 2008), the external capsule (Arfanakis et al., 2002; Bazarian et al., 2007; Bazarian et al., 2011), the centrum semiovale (Grossman et al., 2011; Holli et al., 2010b; Inglese et al., 2005; Miles et al, 2008), inferior fronto-occipital fasciculus (Cubon et al., 2011; Messe et al., 2011; Singh et al., 2010; Smit et al., 2011), inferior longitudinal fasciculus (Cubon et al., 2011; Messe et al., 2011; Niogi et al., 2008a&b; Singh et al., 2010), superior longitudinal fasciculus (Cubon et al., 2011; Geary et al., 2010; Mayer et al., 2010; Matthews et al., 2011; Niogi et al., 2008a&b), uncinate fasciculus (Geary et al., 2010; Mayer et al., 2010; Niogi et al., 2008a&b; Singh et al., 2010), corona radiata (Little et al., 2010; Maruta et al., 2010; Matthews et al., 2011; Mayer et al., 2010; Niogi et al., 2008a&b), corticospinal tract (Henry et al., 2011; Messe et al., 2011; Singh et al., 2010), the cingulum bundle (MacDonald et al., 2011; Niogi et al., 2008a&b; Rutgers et al., 2008a), forceps minor and major (parts of corpus callosum; Messe et al., 2011), forceps major (Little et al., 2010; Messe et al., 2011), cerebral lobar white matter (Lipton et al., 2009; Rutgers et al., 2008a), mesencephalon (Hartikainen et al., 2010; Holli et al., 2010b), the sagittal stratum (Cubon et al., 2011; Geary et al., 2010;), frontal lobe, parietal lobe, temporal lobe, occipital lobe (Salmond et al., 2006), dorsolateral prefrontal cortex (Lipton et al., 2009; Zhang et al., 2010), cerebellar peduncles (MacDonald et al., 2011; Maruta et al., 2010), hippocampus (Singh et al., 2010), fornix (Singh et al., 2010), thalamus (Grossman et al., 2011), thalamic radiation (Cubon et al., 2011; Messe et al., 2011), orbitofrontal white matter (MacDonald et al., 2011), subcortical white matter (Lipton et al., 2008), fronto-temporo-occipital association fiber bundles (Rutgers et al., 2008a), acoustic radiation (Cubon et al., 2011), and deep cortical brain regions (Brandstack et al., 2011).
In summary, there is a great deal of variability in DTI studies of mTBI with respect to the time period of scanning at post-injury, as well as other factors including magnet strength, brain regions examined, and methods of analyses, as noted above. Further, differences in both anatomical location of observed brain alterations, as well as in the nature of these alterations (i.e., increased versus decreased FA), all contribute to the difficulty in interpreting this body of data. The findings are nonetheless striking in that they all suggest that radiological evidence supports small and subtle brain injuries in mTBI (see Table 3 and 4 for details). This evidence would not be possible if conventional MRI and CT scans alone were used to establish brain injury; it takes more advanced and sophisticated methods such as DTI that are sensitive to diffuse axonal injury to delineate these abnormalities. Most of the studies reviewed, however, were cross-sectional studies, with scans acquired at different times post-injury. The cross-sectional nature of most of these studies further highlights the need for longitudinal studies that follow a group of mTBI patients from early in the course of injury, i.e., the first 24 to 72 hours, with repeat scans at several weeks, 1 month, 3 months, 6 months, and 1 year. Such data would provide important information regarding the reversal of damage as well as provide important information about what radiological features early in the course of injury predict good outcome versus poorer outcome with concomitant post-concussive symptoms. There is also a clear need for individualized imaging biomarkers (personalized medicine approach), where a single subject could be compared to a normative group for accurate diagnosis, with MR biomarkers also providing information about prognosis in order to implement treatment plans. These and other issues are the topic of future directions of research, which follows.
Future Directions of Research
Summary
Until quite recently no sufficiently robust radiological technologies existed, on either the acquisition or post-processing side, to detect small and subtle brain alterations that are extant in mTBI (see also Irimia et al., 2011). Today, there are advanced image acquisition sequences available to identify and to quantify small regions of extra- and intra-cortical bleeding (i.e., SWI), as well as diffuse axonal injuries (i.e., DTI). These advanced imaging tools are particularly important for investigating brain pathology in mTBI, where conventional MRI and CT imaging have failed to provide radiological evidence of brain injuries. Hence we are now able to detect and to localize brain alterations in mTBI, which is a critical first step, as it means that the diagnosis of mTBI can be based on radiological evidence, rather than symptoms alone. We are now also able to evaluate the extent of brain injuries. Moreover, we have the capability to observe changes post-injury, and longitudinally, to determine prognosis based on early and intermediate stages of brain injury. The latter may lead to the early identification of those individuals who are likely to recovery versus those who are more likely to experience prolonged postconcussive symptoms.
Thus a focus on longitudinal studies is needed to understand the dynamic nature of brain injuries change over time, as there is very little information about the different patterns of recovery versus non-recovery, and how these changes are reflected by a given imaging modality. For example, lesions may be visible using one imaging modality but not another, depending upon the pattern and stage of recovery. Consequently, the combined use of multimodal imaging, using semi-automated measures for analyses, and including a longitudinal focus would greatly further our understanding of mTBI. These and other future directions for research are elaborated upon below.
Multi-modal imaging and standardized protocols
What is needed is the establishment of acquisition protocols that include multimodal imaging to characterize brain alterations in mTBI, as one imaging modality may not accurately capture brain alterations in mTBI. Both Duhaime and coworkers (2010) and Haacke and coworkers (2010) also discuss the need for more standardized image acquisition protocols for both research and for clinical purposes. It is important also to select optimal imaging acquisition protocols that characterize the kind of injury that is present in mTBI. This approach has been used successfully in research in Alzheimer’s disease, multiple sclerosis, and in psychiatric disorders such as schizophrenia (e.g., Leung et al., 2009; Zou et al., 2005). Following these principles is the Clinical Consortium on PTSD and TBI (INTRuST; see details: http://intrust.spl.harvardu.edu/pages/imaging and see http://intrust.sdsc.edu/), a new initiative sponsored by the Department of Defense, which includes the acquisition of multimodal imaging, from six multi-national centers, using a high resolution, multi-shell DTI protocol as well a SWI and resting-state fMRI acquisition sequences, in addition to anatomical MRI sequences.
More specific measures of microstructural abnormalities
With regard to diffusion imaging, future advances will likely include more specific parameters than FA and MD. For example, free-water is a measure that can be derived from conventional DTI to increase the specificity of observed microstructural abnormalities. The free-water model (Pasternak et al., 2009) assumes that there are two distinct compartments, one that is comprised of water molecules that are freely diffusing in extracellular space, and another that is comprised of the remaining molecules, which are restricted by the cellular tissue. As a result, it is possible to estimate explicitly the volume of extracellular water, a measure that is sensitive to vasogenic edema, and therefore consequently likely specific to neuroinflammation as well. In addition, DTI indices such as FA and MD that are corrected for free-water can be used to estimate the properties of the remaining compartment, i.e., cellular tissue. These corrected values are tissue specific (Pasternak et al., 2009, 2011c) and the correlation between corrected FA and corrected MD is reduced (Metzler-Badley et al., 2012), which makes it possible to use these two parameters, separately, to evaluate tissue degeneration versus extracellular swelling (vasogenic edema and inflammation) and cell swelling (cytotoxic edema). In the very near future, this free-water method may be utilized to investigate and to track brain injury over time and to establish possible biomarkers that predict outcome based on knowing the type (vasogenic versus cytotoxic), location, and extent of the edema.
Multi-shell diffusion weighted imaging (DWI) and kurtosis
Multi-shell diffusion imaging is another new approach that may be useful in future imaging studies of mTBI. Here, DWI is acquired at multiple b-values in the same session and it provides additional microstructural information about the organization of white and gray matter. In these scan sequences, b-values, which are higher than the standard DTI acquisition (e.g., 3000mm/s2), are used to obtain diffusion properties that vary with different gradient strengths. Diffusion Kurtosis Imaging (DKI), a technique that uses multi-shell diffusion imaging, measures the non-Gaussian behavior of water diffusion, and has been shown to be sensitive to characterizing subtle changes in neuronal tissue (Jenkins et al., 2005).
The kurtosis measure is likely more sensitive to biological processes such as “reactive astrogliosis” compared with the more general measures of FA and MD (Zhou et al., 2012). Specifically, kurtosis is a measure of the deviation from the diffusion tensor model (Gaussian diffusion), and thus complements the other measures that are derived from the tensor model. For example, the directionally specific kurtosis measure has been shown to be more sensitive to developmental changes in white and gray matter than FA or MD in rat brains (Cheung, et al 2009). Thus, in this way, kurtosis is more sensitive than traditional diffusion measures to changes in restricted diffusion and thereby has the potential to detect changes related to cells (i.e., astrocytes, neurons, and oligodendrocytes), as opposed to less specific measures of changes in the integrity of tissue (e.g., FA and MD).
Lack of homogeneity in brain trauma and the need for a personalized medicine approach
While we have only touched the surface with respect to what more sensitive imaging technology can inform us about mTBI, another area of interest for future research is to go beyond group analyses, i.e., comparing a group of patients with mTBI with a group of normal controls. Group analyses, in fact, are somewhat misleading because they are predicated on finding larger differences between groups than within groups. Unfortunately, brain trauma is a very heterogeneous disorder, and group analyses, particularly those that use whole brain analyses (i.e., comparing average mTBI brain to average MRI brain), are not well suited for accurate analysis because they obscure the individual differences that characterize brain injuries. The heterogeneity of brain trauma is a topic that is also reviewed in this issue by Dr. Michael Lipton and colleagues.
To circumvent this heterogeneity, a personalized approach needs to be developed that can be incorporated into comparisons between mTBI and normal controls. Such an approach must go beyond a visual inspection of DTI maps such as FA and ADC, to a quantitative, objective approach. The latter may then be used as both radiological evidence of brain injury and to provide an individual profile of brain injury, as well as to form the basis for comparing normal controls and patients with mTBI.
One possible approach to this problem is to build normative atlases based on normal controls, along with robust statistics (e.g., z-score and receiver operator-ROC- curves) to detect “out-of-the-normal” imaging features (e.g., FA, MD, free-water, and kurtosis) at various locations in the brain. This information may then be used to detect brain regions that are abnormal in any of these measures, leading to a diagnosis of diffuse axonal injury (FA, MD), or edema (free water), or myelin damage (kurtosis), which would be specific for each individual case. In this way, one could not only establish a profile of injury for each individual patient, but one could also perform group analyses based on the severity and load of injuries (i.e., extent and number of brain regions involved), without relying on the assumption of a common pattern of injury location among all patients with TBI. The latter assumption is the current approach to group analyses including brain-wise, voxel-based analyses that create averages of brain regions, i.e., comparing a group of healthy and injured brains simultaneously, and demonstrating where in the brain for the entire group of mTBI (not each individual), differs from a group of healthy controls.
Figure 8 shows an example of one mTBI case compared to a normative atlas of FA and of MD, where z-score maps highlight brain regions that are more than three standard deviations from the normative brain atlas for FA (top of figure) and MD (bottom of figure). This is the kind of information that can be evaluated for individual cases, where normative atlases can be built to examine any number of measures (i.e., FA, MD, free-water, kurtosis, etc.).
Figure 8.
Z-score maps for a patient with chronic mTBI subject. Z-score maps were created from a comparison to a normative atlas. The regions in red and yellow show statistically significant abnormal regions for either FA (top) or MD (bottom).
A further example is shown in Figure 9, which depicts more than three standard deviations from the normative brain atlas for free-water, where subject specific maps were created to show abnormal free-water (edema) in two individuals. The individual on the top shows more localized abnormalities, while the individual on the bottom shows more small, scattered lesions that have an edematous or neuroinflammatory component (Pasternak et al., 2011b). These findings may vary across subjects, from localized pathologies to having a more diffuse and scattered pattern of pathology, as seen here, and, importantly, provide information about individual pathology that is not detected using conventional MR or CT. These variations pose extreme challenges to group comparisons, though group comparisons may still be made. They also highlight the heterogeneity that is characteristic of TBI, including mTBI.
Figure 9.
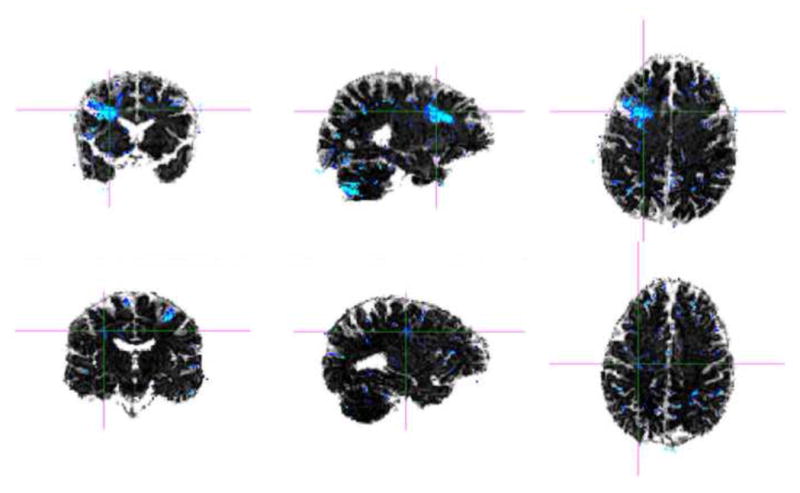
Z-score maps for two patients with chronic mTBI. Z-score maps were created from a comparison to a normative atlas. The images at the top show a patient with more localized regions of increased free-water while the images at the bottom show a patient with more diffuse regions of increased free-water (blue color indicates statistically significant areas of free-water compared with the normative atlas).
Tractography
Another promising method for probing alterations in white matter in mTBI is tractography. The word tractography refers to any method for estimating the trajectories of the fiber tracts (bundles) in the white matter. Many methods have been proposed for tractography, and the results will vary depending on the chosen method. For example, deterministic tractography involves directly following the main diffusion direction, whereas probabilistic methods estimate the likelihood of two regions being connected (Bjornemo et al., 2002; Behrens et al., 2003). The most common deterministic approach is streamline tractography (see Figure 10) (Basser et al., 2000; Conturo et al., 1999; Mori et al. 1999; Westin et al., 1999), which is closely related to an earlier method for visualization of tensor fields known as hyperstreamlines (Delmarcelle et al. 1992). For a clinical and technical overview of tractography in neurological disorders, the reader is referred to Ciccarelli et al. (2008). For reviews of tractography techniques including explanations of common tractography artifacts and a comparison of methods, the reader is referred to Jones (2008) and Lazar (2010).
Figure 10.
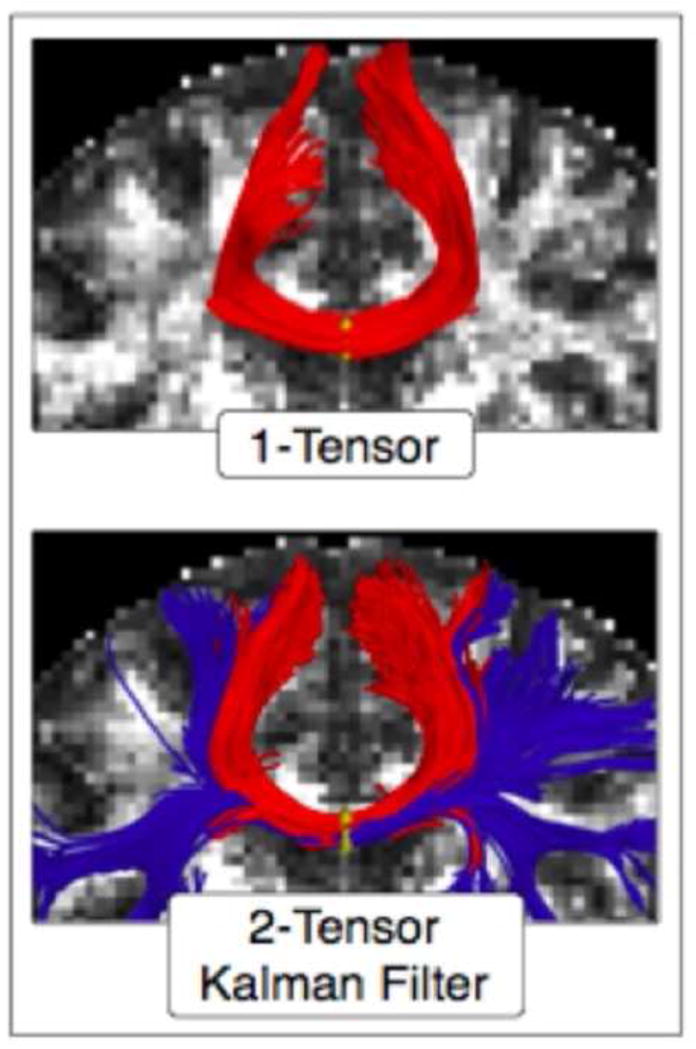
Part of the corpus callosum fibers, where seeding was done in the mid-sagittal plane of the corpus callosum. Top figure shows the tracing using the standard single-tensor model and bottom figure shows tracts generated with the two-tensor model.
DTI based streamline tractography has been used by several investigative groups to examine schizophrenia and other neurological and psychiatric disorders (see review in Kubicki et al., 2007; Shenton et al., 2010; Whitford et al., 2011). The main limitation of this method is that it does not allow one to trace fibers through complex fiber orientations such as branching and crossing fibers. This is because DTI based streamline tractography assumes that there exists only one fiber bundle oriented coherently in a single direction at each voxel. Thus voxels where different fiber bundles cross cannot be characterized using this model. Consequently, several advanced models have been proposed in the literature, such as a high-order tensor model (Ozarsian et a., 2003; Barmpoutis et al., 2009), a spherical harmonics based nonparametric model (Anderson et al., 2005) and a multi-tensor model (Tuch et al., 2002; Pasternak et al., 2008; Malcolm et al., 2010), among others.
Figure 10 shows a method of tracing the corpus callosum fiber bundle using single tensor DTI based tractography (on the left) and another method using two-tensor DTI based tractography (on the right) that illustrates the improved fiber tracking using the two-tensor method. This method makes it possible now to trace multiple fiber tracts that traverse the brain and connect several different brain regions (e.g., Malcolm et al., 2010; Pasternak et al., 2008; Tournier et al., 2008).
Figure 11 shows all of the fibers in the brain that travel through the corpus callosum, in a single case, using two-tensor tractography (Malcolm et al., 2010). Of note here, the methodology for analysis can remain the same as before, i.e., trace a certain fiber bundle in healthy controls and compute the “typical” diffusion properties such as FA, MD, etc., then trace the same bundle in TBI subjects, and finally compare them to detect differences. What is different in terms of moving from a single tensor model to two and multi-tensor models is that it improves the ability to accurately identify crossing fiber tracts.
Figure 11.
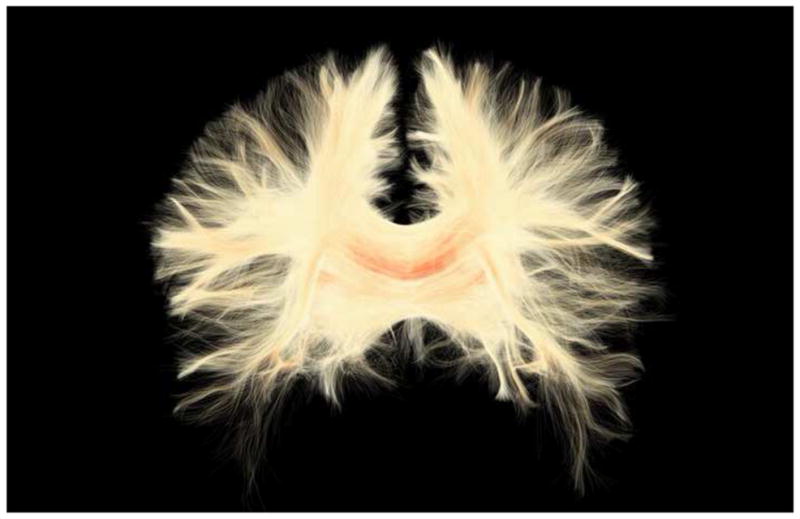
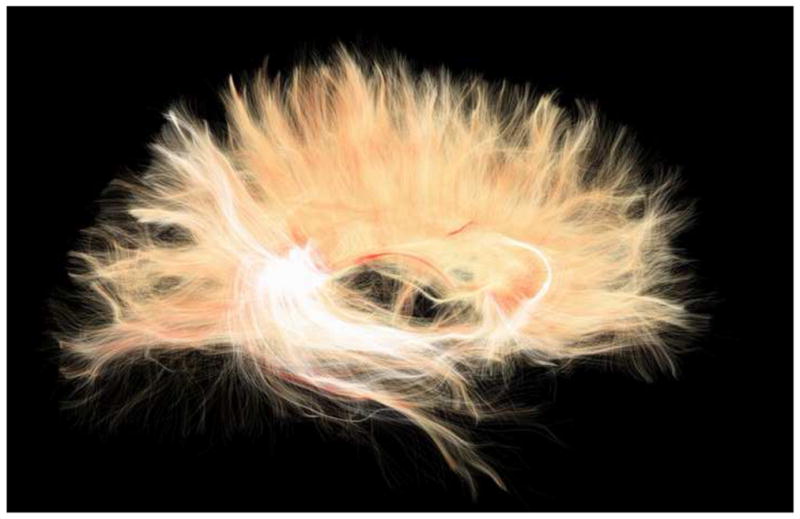
Panel A shows a coronal view of white matter fiber tracts using a two-tensor model that go through the corpus callosum Panel B is a sagittal view of the corpus callosum shown in Panel A.
Mean-squared-displacement and return-to-origin probabilities
Other more sensitive markers of diffusion, such as mean-squared-displacement and return-to-origin probabilities, can be obtained from more advanced diffusion scans such as multi-shell (multiple b-values) diffusion imaging (e.g., Mitra, 1992; Yu-Chien et al., 2007). Although the scan time for these acquisitions is longer, they provide more subtle information beyond what can be obtained from DTI as it is currently used. For example, the return-to-origin probability measures the probability that a water molecule will return to its starting point in a given amount of time. This measure will be higher for white matter due to restriction on the motion of water as a result of the coherent layout of the white matter fibers. This probability will be lower in gray matter and lowest in CSF, where water is essentially free to move. Thus this measure captures subtle changes in the organization of white matter and has been shown to be more sensitive to de-myelination of white matter tracts compared to DTI measures such as FA (Assaf et al., 2005).
Another diffusion measure that is more sensitive to the restriction of water molecules due to cellular boundaries is mean-square-displacement (MSD)(e.g., Assaf et al., 2002). This measure is the mean distance travelled by a water molecule in a given diffusion time. Thus, MSD is highly dependent on the cellular structure at any given location and hence could be related to the concentration of certain biochemicals. This could be validated by correlating this measure with the concentration of certain biochemicals such as phospholipids as obtained using an MRS scan. Hence, such measures can be very useful for detecting mTBI since the underlying tissue changes are often very subtle and may be missed by current DTI and DKI imaging protocols. In the near future, this approach could also be combined with other brain biomarkers that are based, for example, on blood-serum, the latter a powerful complementary tool that provides information about genes and proteins affected by brain injury (see section below; see also Mondello et al., 2011).
Identification and delineation of brain injury, and the development of biomarkers for possible treatment and treatment efficacy trials
Advanced multi-modal neuroimaging techniques provide radiological evidence of mTBI that go beyond self-report and other more conventional measures, and may elucidate further the mechanisms and neurophysiology underlying mTBI. This is an important first step. More studies, however, are needed using multi-modal imaging techniques. Further, based on their enhanced sensitivity, many of these more advanced techniques should be used to monitor treatment efficacy, and serve as endpoints for new trials of medication aimed at neuroplasticity or neuroinflammation in TBI.
One example of multi-modal neuroimaging is to combine MRS and DTI in the same subjects. MRS and DTI are highly complimentary given the biochemical and structural focus of each modality. However, few studies have utilized the two together. In severe head injury, the combination of these two modalities has provided greater diagnostic accuracy for predicting outcome one year following injury (Tollard et al., 2009), than either MRS or DTI alone. It is likely that this same combination would also provide greater sensitivity to the more subtle changes in mTBI.
The co-localization of DTI and MRS may also provide greater insight into the underlying physiological changes that occur in mTBI. An early MRS study by Cecil and coworkers (Cecil et al., 1998) did not have DTI available at the time, but their findings of decreased N-acetyl aspartate (NAA; a putative neuronal and axonal marker; see review in this issue by Lin et al.) in the corpus callosum corroborates DTI findings of reduced FA, as previously described in the current review. As NAA is transported down axons and the loss of FA is attributed to axonal injury, a strong correlation between these two measures further strengthens the argument for axonal injury. NAA MRS measures can also help to validate DTI studies in regions of fiber crossings and confirm that decreased FA can be attributed to loss of fiber integrity as opposed to technical issues. Studies of schizophrenia (e.g., Tang et al., 2007) and motor neuron disease (Nelles et al., 2008) have also utilized MRS and DTI in this complimentary fashion.
Additionally, by combining different biomarkers from blood with MR biomarkers, we may be able to delineate further specific types of brain injury involved in mTBI, as well as how they change over time, and what the evolution is of secondary damage or progression of types of injury over time. More specifically, serum biomarker profiles, as an outcome measure of brain damage, are being used to detect brain damage in ischemic stroke and TBI in animal studies (Liu et al, 2010). This approach may also be useful in humans where TBI-specific biomarkers could be developed, based on proteomics, to provide distinctive profiles of specific genes and proteins that are altered in mTBI (e.g., Mondello et al., 2011). The free-water model, described above, could also be combined with measures of proteins involved in neuroinflammation in the brain sto develop combined TBI-specific biomarkers that may detect brain injuries and predict course, outcome, and treatment response better than using either a proteomic or MR biomarker alone.
Thus combining multimodal imaging modalities, and combining genomic and proteomic biomarkers with MR biomarkers, may lead to the discovery of more specific biomarkers of biochemical and physiological processes, which, in turn, may provide important new information about primary and secondary consequences of injury, and assist in determining what combination of biomarkers are indicative of axonal injury, inflammation, demyelination, apoptosis, neuroregeneration, etc., and what combination best predict outcome and treatment. It is also likely that the development of multiple biomarkers will lead to a new stratification of patients based on several biomarkers that, when combined, are more specific to important measures of brain injury than is any one biomarker alone. This new approach to developing multi-modal MR biomarkers and combining these with serum based proteomic biomarkers to investigate brain injuries in mTBI may go a long way to providing more accurate diagnosis of mTBI, as well as providing important indicators of treatment response and outcome.
Acknowledgments
This work was supported in part by the INTRuST Posttraumatic Stress Disorder and Traumatic Brain Injury Clinical Consortium funded by the Department of Defense Psychological Health/Traumatic Brain Injury Research Program (X81XWH-07-CC-CS-DoD; MES, JSS, SB, OP, MK, YR, M-AV, C-FW, RZ), by an NIH NINDS funded R01 (R01 NS 078337; RS, MES, JSS), by a Center for Integration of Medicine (CIMIT) Soldier in Medicine Award (SB, MES), by an NIH NIMH funding R01 (R01 MH082918; SB), by funding from the National Research Service Award (T32AT000051; MPP) from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institute of Health, by the Harvard Medical School Fellowship as part of the Eleanor and Miles Shore Fellowship Program (HH), by the Deutsche Akademischer Austauschdienst (DAAD; IK), and by funding from NCRR, including the National Alliance for Medical Image Computing (NAMIC-U54 EBOO5149; RK, MK, MES), and the Neuroimaging Analysis Center (NAC; P41RR13218; RK and CF).
BIBLIOGRAPHY
- Adams JH, Doyle D, et al. Diffuse axonal injury in head injury: definition, diagnosis, and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Alexander MP. Mild TBI: pathophysiology, natural history, and clinical management. Neurology. 1995;45(7):253–260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- Anderson A. Measurement of fiber orientation distributions using high angular resolution diffusion imaging. Magn Res Med. 2005;54(5):1194–1206. doi: 10.1002/mrm.20667. [DOI] [PubMed] [Google Scholar]
- Anderson CV, Bigler ED, et al. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain-injured patients. J Clin Exp Neuropsych. 1995;17:900–908. doi: 10.1080/01688639508402438. [DOI] [PubMed] [Google Scholar]
- Anderson CV, Wood DM, et al. Lesion volume, injury severity, and thalamic integrity following head injury. J Neurotrauma. 1996;13(2):59–65. doi: 10.1089/neu.1996.13.59. [DOI] [PubMed] [Google Scholar]
- Arciniegas DB, Anderson CA, et al. Mild traumatic brain injury: A neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat. 2005;1:311–327. [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- Ashwal S, Holshouser BA, et al. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev Neurosci. 2006;28:309–326. doi: 10.1159/000094157. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Ben-Bashat D, et al. High b-value q-space analyzed diffusion-weighted MRI: application to multiple sclerosis. Magn Reson Med. 2002;47(1):115–126. doi: 10.1002/mrm.10040. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Chapman J, Ben-Bashat D, et al. White matter changes in multiple sclerosis: correlation of q-space diffusion MRI and 1-H MRS. Mag Res Imaging. 2005;23(6):703–710. doi: 10.1016/j.mri.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Babikian T, Freier MC, et al. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Babikian T, Freier MC, et al. Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr Neurol. 2005;33(3):184–194. doi: 10.1016/j.pediatrneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G, Houda DA, et al. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Barmpoutis A, Hwang MS, Howland D, et al. Regularized positive-definite fourth order tensor field estimation from DW-MRI. NeuroImage. 2009;45(1):S153–S162. doi: 10.1016/j.neuroimage.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, et al. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT–MRI data. Mag Res Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, et al. Bench to bedside: evidence for brain injury after concussion--looking beyond the computed tomography scan. Acad Emerg Med. 2006;13(2):199–214. doi: 10.1197/j.aem.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, et al. DTI detects clinically important axonal damage after mild TBI: a pilot study. J Neurotrauma. 2007;24(9):1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, et al. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2011 Nov 11; doi: 10.1016/j.mri.2011.10.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Wong T, et al. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999;13(3):173–189. doi: 10.1080/026990599121692. [DOI] [PubMed] [Google Scholar]
- Behrens T, Woolrich MM, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Mag Res Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Belanger HD, Kretzmer T, et al. Symptom complaints following combat-related traumatic brain injury: Relationship to traumatic brain injury severity and posttraumatic stress disorder. J Int Neuropsychol Soc. 2009;16:194–199. doi: 10.1017/S1355617709990841. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD, et al. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19(1):5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bell RS, Loop JW. The utility and futility of radiographic skull examination for trauma. N Engl J Med. 1971;284(5):236–239. doi: 10.1056/NEJM197102042840504. [DOI] [PubMed] [Google Scholar]
- Benson RR, Meda SA, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in TBI. J Neurotrauma. 2007;24(3):446–459. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- Bergeson AG, Lundin R, et al. Clinical rating of cortical atrophy and cognitive correlates following traumatic brain injury. Clin Neuropsychol. 2004;18(4):509–520. doi: 10.1080/1385404049052414. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc. 2004;10(5):794–800. doi: 10.1017/S1355617704105146. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14(1):1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, et al. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 1997;18(1):11–23. [PMC free article] [PubMed] [Google Scholar]
- Bjornemo M, Brun A, Kikinis R, Westin C-F. Regularized stochasticwhite matter tractography using diffusion tensor MRI. Medical Image Computing and Computer Assisted Intervention (MICCAI) 2002:435–442. [Google Scholar]
- Blumbergs PC, Scott G, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344(8929):1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- Bouix S, Pelavin P, et al. Diagnosis of Diffuse Axonal Injury with Diffusion Tensor Imaging. The 3rd Federal Interagency Conference on TBI; Washington DC. 2011. [Google Scholar]
- Brandstack N, Kurki T, et al. Diffusivity of normal-appearing tissue in acute traumatic brain injury. Clin Neuroradiol. 2011;21(2):75–82. doi: 10.1007/s00062-011-0058-5. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Friedman SD, et al. Magnetic resonance spectroscopy in TBI. J Head Trauma Rehabil. 2001;16(2):149–164. doi: 10.1097/00001199-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Bruns JJ, Jagoda A. Mild Traumatic Brain Injury. Mt Sinai J Med. 2009;76:129–137. doi: 10.1002/msj.20101. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57(4):688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, et al. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134(8):2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Injury, Prevention, & Control: Traumatic Brain Injury. Center for Disease Control and Prevention; 2010. http://www.cdc.gov/traumaticbraininjury/statistics.html. [Google Scholar]
- Carroll LJ, Cassidy JD, et al. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004a;43(Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, et al. Systematic search and review procedures: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004b;43(Suppl):11–14. doi: 10.1080/16501960410023660. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Hills EC, Sandel ME, et al. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 1998;88(5):795–801. doi: 10.3171/jns.1998.88.5.0795. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, et al. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. The Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Cohen BA, Inglese M, et al. Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. AJNR Am J Neuroradiol. 2007;28(5):907–913. [PMC free article] [PubMed] [Google Scholar]
- Coles JP. Imaging after brain injury. Br J Anaesth. 2007;99(1):49–60. doi: 10.1093/bja/aem141. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, et al. Tracking Neuronal Fiber Pathways in the Living Human Brain. Neurobiology. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubon VA, Putukian M. A Diffusion Tensor Imaging Study on the White Matter Skeleton in Individuals with Sports-Related Concussion. J Neurotrauma. 2011;28(2):189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, et al. Diffuse and sptially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage. 2011 Oct 20; doi: 10.1016/j.neuroimage.2011.10.050. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Delmarcelle T, Hesselink L. Visualization of second order tensor fields and matrix data. Proceedings IEEE Visualization. 1992:316–323. [Google Scholar]
- Ding K, Marquez de Plata C, et al. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J Neurotrauma. 2008;25:1433–1440. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhaime AC, Gean AD, et al. Common data elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1661–1666. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- Eisenberg HM, Levin HS. Computed tomography and magnetic resonance imaging in mild to moderate head injury. In: Levin HS, Eisenberg HM, Benton AL, editors. Mild Head Injury. NY: Oxford University Press; 1989. pp. 133–141. [Google Scholar]
- Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Res Med. 2006;55(1):136–46. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, et al. The activation of attentional networks. NeuroImage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Faul MD, Xu L, et al. TBI in the United States: emergency department visits, hospitalizations, and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Fitzgerald DB, Crosson BA. Diffusion weighted imaging and neuropsychological correlates in adults with mild traumatic brain injury. Int J Psychophysiol. 2011;82(1):79–85. doi: 10.1016/j.ijpsycho.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe AC, et al. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fujiwara E, Schwartz ML, et al. Ventral frontal cortex functions and quantified MRI in traumatic brain injury. Neuropsychologia. 2008;46:461–474. doi: 10.1016/j.neuropsychologia.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Baxter L, et al. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J Neurol Neurosurg Psychiatry. 2005;76(7):984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Johnson SC, et al. Nonspecific white matter degeneration following traumatic brain injury. J Int Neuropsychol Soc. 1995;1(1):17–28. doi: 10.1017/s1355617700000060. [DOI] [PubMed] [Google Scholar]
- Garnett MR, Blamire AM, et al. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;120(10):2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Geary EK, Kraus MF, et al. Verbal learning differences in chronic mild traumatic brain injury. J Int Neuropsychol Soc. 2010;16(3):506–516. doi: 10.1017/S135561771000010X. [DOI] [PubMed] [Google Scholar]
- Gentry LR. Imaging of closed head injury. Radiology. 1994;191:1–17. doi: 10.1148/radiology.191.1.8134551. [DOI] [PubMed] [Google Scholar]
- Gentry LR, Godersky JC, et al. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150(3):663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- Goldstein M. Traumatic brain injury: a silent epidemic (Editorial) Ann Neurol. 1990;27:327. doi: 10.1002/ana.410270315. [DOI] [PubMed] [Google Scholar]
- Green R, Koshimori Y, et al. Research digest. Understanding the organic basis of persistent complaints in mTBI: findings from functional and structural neuroimaging. Neuropsychol Rehabil. 2010;20(3):471–478. doi: 10.1080/09602011003693298. [DOI] [PubMed] [Google Scholar]
- Greiffenstein M. Clinical myths of forensic neuropsychology. Clin Neuropsych. 2008:1–11. doi: 10.1080/13854040802104873. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Ge Y, et al. Thalamus and cognitive impairment in Mild Traumatic Brain Injury: A diffusional kurtosis imaging study. J Neurotrauma. 2011 Sep 15; doi: 10.1089/neu.2011.1763. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Duhaime, et al. Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging. 2010;32(3):516–543. doi: 10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Mittal S, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30(1):19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Xu Y, et al. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- Hackney DB. Skull radiography in the evaluation of acute head trauma: a survey of current practice. Radiology. 1991;181(3):711–714. doi: 10.1148/radiology.181.3.1947086. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Waljas M, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J Clin Exp Neuropsychol. 2010;32(7):767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Dixon CE. Neurochemical changes in mild head injury. Semin Neurol. 1994;14:25–31. doi: 10.1055/s-2008-1041055. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28(10):2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Himanen L, Portin R, et al. Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Inj. 2005;19:93–100. doi: 10.1080/02699050410001720031. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, et al. Mild TBI in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Goldberg HM, et al. Care of war veterans with mild traumatic brain injury--flawed perspectives. N Engl J Med. 2009;360(16):1588–1591. doi: 10.1056/NEJMp0810606. [DOI] [PubMed] [Google Scholar]
- Holli KK, Harrison L, et al. Texture analysis of MR images of patients with mild traumatic brain injury. BMC Med Imaging. 2010a;10:8. doi: 10.1186/1471-2342-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holli KK, Wäljas M, et al. Mild traumatic brain injury: tissue texture analysis correlated to neuropsychological and DTI findings. Acad Radiol. 2010b;17(9):1096–1102. doi: 10.1016/j.acra.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Holshouser BA, Tong KA, et al. Proton MR spectroscopic imaging depicts diffuse axonal injury in children with TBI. AJNR Am J Neuroradiol. 2005;26(5):1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Huang M, Theilmann R, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma. 2009;26(8):1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Hughes DG, Jackson A, et al. Abnormalities on magnetic resonance imaging seen acutely following brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiol. 2004;46:550–558. doi: 10.1007/s00234-004-1227-x. [DOI] [PubMed] [Google Scholar]
- Huisman TA, Schwamm LH, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- Hunter JV, Wilde EA, et al. Emerging Imaging Tools for Use with Traumatic Brain Injury Research. J Neurotrauma. 2011 Oct 17; doi: 10.1089/neu.2011.1906. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M, Makani S, et al. Diffuse axonal injury in mild TBI: a diffusion tensor imaging study. J Neurosurgm. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Irimia A, Chambers MC, et al. Comparison of acute and chronic traumatic brain injury using semi-automatic multimodal segmentation of MR volumes. J Neurotrauma. 2011;(11):2287–2306. doi: 10.1089/neu.2011.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. 2005;18(3):301–317. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Lovell MR, et al. Prevalence of abnormal CT-scans following mild head injury. Brain Inj. 2000;14(12):1057–1061. doi: 10.1080/02699050050203559. [DOI] [PubMed] [Google Scholar]
- Jang SH. Diffusion tensor imaging studies on corticospinal tract injury following traumatic brain injury: A review. Neuro Rehabi,l. 2011;29(4):339–345. doi: 10.3233/NRE-2011-0710. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Teasdale G, et al. Brain lesions detected by magnetic resonance imaging in mild to severe head trauma. Lancet. 1986;2:445–646. doi: 10.1016/s0140-6736(86)92145-8. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Johnston KM, Ptito A, et al. New frontiers in diagnostic imaging in concussive head injury. Clin J Sport Med. 2001;11(3):166–175. doi: 10.1097/00042752-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kou Z, Tong KA, et al. The role of advanced MR imaging findings as biomarkers of TBI. J Head Trauma Rehabil. 2010;25(4):267–282. doi: 10.1097/HTR.0b013e3181e54793. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23(7):675–685. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF, Kurland LT. The epidemiology of neurologic disease. In: Joynt RJ, editor. Clinical Neurology. Philadelphia: JB Lippincott; 1993. rev Ch. 66. [Google Scholar]
- Lange RT, Iversion GL, et al. Diffusion tensor imaging findings are not strongly associated with postconcussional disorder 2 months following mild traumatic brain injury. J Head Trauma Rehabil. 2011 Jun 2; doi: 10.1097/HTR.0b013e318217f0ad. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lazar M. Mapping brain anatomical connectivity using white matter tractography. NMR Biomed. 2010;23:821–835. doi: 10.1002/nbm.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TH, Gean AD. Neuroimaging of traumatic brain injury. Mt Sinai J Med. 2009;76(2):145–162. doi: 10.1002/msj.20102. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q. 1991;7(1):1–30. [PubMed] [Google Scholar]
- Leung KK, Clarkson MJ, et al. Robust atrophy rate measurement in Alzheimer’s disease using multi-site serial MRI: tissue-specific intensity normalization and parameter selection. Neuroimage. 2010;50(2):516–523. doi: 10.1016/j.neuroimage.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Matiss S, et al. Neurobehavioral outcome following head injury: a three center study. J Neurosurg. 1984;66:234–243. doi: 10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- Levin HS, Amparo E, et al. Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg. 1987;66(5):706–713. doi: 10.3171/jns.1987.66.5.0706. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde E, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27(4):683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Levine B, Kovacevic N, et al. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70:771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Lin A, Ross BD, et al. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2005;2(2):197–214. doi: 10.1602/neurorx.2.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Ramadan S, et al. Radiological Society of North America. Chicago, IL: 2010. Neurochemical Changes in Athletes with Chronic Traumatic Encephalopathy. [Google Scholar]
- Lipton ML, Gellella C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25(11):1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252(3):816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- Lishman WA. Physiogenesis and psychogenesis in the ‘post-concussional syndrome’. Br J Psychiatry. 1988;153:460–469. doi: 10.1192/bjp.153.4.460. [DOI] [PubMed] [Google Scholar]
- Little DM, Kraus MF, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74(7):558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY, Maldjian AJ, et al. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol. 1999;20(9):1636–1641. [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Akinyi L, Scharf D, et al. Ubiquitin C-terminal hydroslase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur J Neurosci. 2010;31:722–732. doi: 10.1111/j.1460-9568.2010.07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungqvist J, Nilssonn D, et al. Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj. 2011;25(4):370–378. doi: 10.3109/02699052.2011.558038. [DOI] [PubMed] [Google Scholar]
- Lo C, Shifteh K, et al. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J Comput Assist Tomogr. 2009;33(2):293–297. doi: 10.1097/RCT.0b013e31817579d1. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Bergquist TF, et al. Relationship between stress, coping, and postconcussion in a healthy adult population. Arch Clin Neuropsych. 1988;13(5):415–424. [PubMed] [Google Scholar]
- MacDonald CL, Johnson AM, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie JD, Siddiqi F, et al. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002;23(9):1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Malcolm JG, Shenton ME, Rathi Y. Filtered multi-tensor tractography. IEEE Trans on Medical Imaging. 2010;29:1664–1675. doi: 10.1109/TMI.2010.2048121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Thomson RH, et al. Traumatic brain injury, major depression, and diffusion tensor imaging: making connections. Brain Res Rev. 2010;64(1):213–240. doi: 10.1016/j.brainresrev.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Maruta J, Suh M, et al. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil. 2010;25(4):293–305. doi: 10.1097/HTR.0b013e3181e67936. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, et al. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage. 2011;54(Suppl 1):S69–75. doi: 10.1016/j.neuroimage.2010.04.269. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hosoda K, et al. Utility of diffusion tensor imaging in the acute stage of mild to moderate traumatic brain injury for detecting white matter lesions and predicting long-term cognitive function in adults. J Neurosurg. 2011;115(1):130–139. doi: 10.3171/2011.2.JNS101547. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling J, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurol. 2010;74(8):643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Ford JC, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng. 2012;40(1):127–140. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, O’Sullivan MJ, Bells S, et al. How and how not to correct for CSF-contamination in diffusion MRI. NeuroImage. 2012;59:1394–1403. doi: 10.1016/j.neuroimage.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Messe A, Caplain S, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp. 2011;32(6):999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L, Grossman RI, et al. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22(2):115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- Miller L. Neuropsychology and pathophysiology of mild head injury and the postconcussive syndrome: clinical and forensic considerations. J Cogn Rehab. 1996;14:8–23. [Google Scholar]
- Mitra PP. Diffusion propagator as a probe of the structure of porous media. Physical Review Letters. 1992;68(24):3555–3558. doi: 10.1103/PhysRevLett.68.3555. [DOI] [PubMed] [Google Scholar]
- Mittl RL, Garossman RI, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol. 1994;15(8):1583–1589. [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Muller U, Jeromin A, et al. Blood-based diagnostics of traumatic brain injuries. Expert Rev Mol Diagn. 2011;11(1):65–78. doi: 10.1586/erm.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, et al. Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P. Diffusion tensor imaging of mild TBI. J Head Trauma Rehabil. 2010;25(4):241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008a;29(5):967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008b;131(12):3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Nolin P, Heroux L. Relations among sociodemographic, neurologic, clinical, and neuropsychologic variables, and vocational status following mild TBI: a follow-up study. J Head Trauma Rehabil. 2006;21(6):514–526. doi: 10.1097/00001199-200611000-00006. [DOI] [PubMed] [Google Scholar]
- Okie S. Traumatic Brain Injury in the War Zone. New Engl J Med. 2005;352:2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DR. Microscopic lesions in the brain following head injury. J Neurol Neurosurg Psychiatry. 1968;31:299–306. doi: 10.1136/jnnp.31.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozarian ME. Generalized diffusion tensor imaging and analytical relationships between diffusion tensor imaging and high angular resolution diffusion imaging. Magn Res Med. 2003;50(5):955–965. doi: 10.1002/mrm.10596. [DOI] [PubMed] [Google Scholar]
- Park JH, Park SW, et al. Detection of traumatic cerebral microbleeds by Susceptibility-Weighted Image of MRI. J Korean Neurosurg Soc. 2009;46:365–369. doi: 10.3340/jkns.2009.46.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Assaf Y, et al. Variational multiple-tensor fitting of fiber-ambiguous diffusion-weighted magnetic resonance imaging voxels. Magn Reson Imaging. 2008;26(8):1133–1144. doi: 10.1016/j.mri.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Bouix S, et al. Diffusion Imaging Reveals Two Spatially Separable Mechanisms In Mild TBI. The 3rd Federal Interagency Conference on TBI; Washington, DC. 2010. [Google Scholar]
- Pasternak O, Kubicki O, et al. Identification of Neuroinflammation in Mild Traumatic Brain Injuries using a Free-Water Atlas. Annual Meeting of the Organization for the Human Brain Mapping.2011a. [Google Scholar]
- Pasternak O, Sochen N, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, et al. Free Water Modulation of White Matter Integrity Measures - with Application to Schizophrenia. Proc Int Soc Magn Reson Med. 2011b;19:2544. [Google Scholar]
- Pfefferbaum A, Sullivan EV, et al. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24(8):1214–1221. [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Res Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1999;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Bouix S, et al. A hierarchical algorithm for MR brain image parcellation. IEEE Trans Med Imaging. 2007;26(9):1201–1212. doi: 10.1109/TMI.2007.901433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Coburn TH. Morphopathological change associated with mild head injury. In: Levin HS, Eisenberg HM, Benton AL, editors. Mild Head Injury. New York: Oxford University; 1989. pp. 37–52. [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Reichenbach JR, Markus B, et al. High-Resolution MR Venography at 3.0 Tesla. J Comput Assist Tomogr. 2000;24(6):949–957. doi: 10.1097/00004728-200011000-00023. [DOI] [PubMed] [Google Scholar]
- Rimel RW, Giordani B, et al. Disability caused by minor head injury. Neurosurgery. 1981;9(3):221–228. [PubMed] [Google Scholar]
- Ross BD, Ernst T, et al. 1H MRS in acute traumatic brain injury. J Magn Reson Imaging. 1998;8(4):829–840. doi: 10.1002/jmri.1880080412. [DOI] [PubMed] [Google Scholar]
- Ross B, et al. MR spectroscopy of hypoxic brain injury. In: Gillard J, Waldman A, Barker PB, editors. Clinical MR Neuroimaging: Diffusion, Perfusion and Spectroscopy. Cambridge: Cambridge University Press; 2005. pp. 690–705. [Google Scholar]
- Ruff RM, Camenzuli L, et al. Miserable minority: emotional risk factors that influence the outcome of a mild TBI. Brain Inj. 1996;10(8):551–565. doi: 10.1080/026990596124124. [DOI] [PubMed] [Google Scholar]
- Rutgers DR, Fillard P, et al. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008b;29(9):1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, et al. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008a;29(3):514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29(1):117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Scheid R, Preul C, et al. Diffuse axonal injury associated with chronic traumatic brain injury, evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- Scheid R, Ott DV, et al. Comparative magnetic resonance imaging at 1.5 and 3 Tesla for the evaluation of traumatic microbleeds. J Neurotrauma. 2007;24(12):1811–1816. doi: 10.1089/neu.2007.0382. [DOI] [PubMed] [Google Scholar]
- Schonberger M, Ponsford J, et al. The Relationship between age, injury severity, and MRI findings after traumatic brain injury. J Neurotrauma. 2009;26:2157–2167. doi: 10.1089/neu.2009.0939. [DOI] [PubMed] [Google Scholar]
- Seeger U, Klose U, et al. Parameterized evaluation of macromolecules and lipids in proton MR spectroscopy of brain diseases. Magn Reson Med. 2003;49(1):19–28. doi: 10.1002/mrm.10332. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Whitford TJ, et al. Structural neuroimaging in schizophrenia: From methods to insights to treatments. Dialogues Clin Neurosci. 2010;12(3):269–332. doi: 10.31887/DCNS.2010.12.3/mshenton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter L, Tong KA, et al. Proton MRS in acute TBI: role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–1705. doi: 10.1089/neu.2004.21.1693. [DOI] [PubMed] [Google Scholar]
- Singh M, Jeong J, et al. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn Reson Imaging. 2010;28(1):22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, et al. New magnetic resonance imaging techniques for the evaluation of traumatic brain injury. J Neurotrauma. 1995;12(4):573–577. doi: 10.1089/neu.1995.12.573. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smits M, Houston GC, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011;53(8):553–563. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2001;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Sosin DM, Sniejek JE, et al. Incidence of mild and moderate brain injury in the United States. Brain Inj. 1996;10:47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, et al. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. Neuroimage. 2011;54(Suppl):1, S21–29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild TBI. Am J Psychiatry. 2009;166(7):768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- Stern RA, Riley DA, et al. Long-term consequences of repetitive brain trauma: Chronic traumatic encephalopathy. PMR. 2011 doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Strangman GE, O’Neil-Pirozzi TM, et al. Regional brain morphometry predicts memory rehabilitation outcome after traumatic brain injury. Front Hum Neurosci. 2010;4:182. doi: 10.3389/fnhum.2010.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH. Invisible wounds of war: Psychological and cognitive injuries, their consequences and services to assist recovery. Santa Monica: CAO: RAND Corp; 2008. [Google Scholar]
- Tate DF, Bigler ED. Fornix and Hippocampal Atrophy in Traumatic Brain Injury. Learn Mem. 2000;7(6):442–446. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thurman DJ. The epidemiology and economics of head trauma. In: Miller L, Hayes R, editors. Head Trauma: Basic, Preclinical, and Clinical Directions. NY: John Wiley & Sons; 2001. pp. 324–347. [Google Scholar]
- Tollard E, Galanaud D, Perlbarg V, et al. Experience of diffusion tensor imaging and 1H spectroscopy for outcome prediction in severe traumatic brain injury: Preliminary results. Crit Care Med. 2009;37(4):1448–55. doi: 10.1097/CCM.0b013e31819cf050. [DOI] [PubMed] [Google Scholar]
- Tong KA, Ashwal S, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227(2):332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- Tong KA, Ashwal S, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56(1):36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Yeh CH, Calamante F, et al. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42(2):617–624. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Ward MA, et al. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: relationship with duration of coma. J Neurotrauma. 2007;24:766–771. doi: 10.1089/neu.2006.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, et al. Assessment of metabolic brain damage and recovery following mild TBI: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, et al. Long-term morbidities following self-reported mild traumatic brain injury. J Clin Exp Neuropsychol. 2007;29:585–598. doi: 10.1080/13803390600826587. [DOI] [PubMed] [Google Scholar]
- Warner MA, Marquez de la Plata C, et al. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma. 2010b;27(12):2121–2130. doi: 10.1089/neu.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner MA, Youn TS, et al. Regionally selective atrophy after traumatic axonal injury. Arch Neurol. 2010a;67(11):1336–1344. doi: 10.1001/archneurol.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D. Military TBI during Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Westin C-F, Maier SE, Khidhir B, Everett P, Jolesz FA, Kikinis R. Image Processing for Diffusion Tensor Magnetic Resonance Imaging. In Medical Image Computing and Computer 1999 [Google Scholar]
- Whitford TJ, Kubicki M, et al. Diffusion Tensor Imaging, Structural Connectivity, and Schizophrenia. Schizophr Res Treatment. 2011:1–7. doi: 10.1155/2011/709523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Bigler ED, et al. Alcohol abuse and traumatic brain injury: quantitative magnetic resonance imaging and neuropsychological outcome. J Neurotrauma. 2004;21:137–147. doi: 10.1089/089771504322778604. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Bigler ED, et al. Post-traumatic amnesia predicts long- term cerebral atrophy in traumatic brain injury. Brain Inj. 2006;20:695–699. doi: 10.1080/02699050600744079. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Bueler E, et al. Neuroimaging Correlates of Traumatic Brain Injury and Suicidal Behavior. J Head Trauma Rehabil. 2011;26(4):276–289. doi: 10.1097/HTR.0b013e31822251dc. [DOI] [PubMed] [Google Scholar]
- Yount R, Raschke KA, et al. Traumatic brain injury and atrophy of the cingulate gyrus. J Neuropsychiatry Clin Neurosci. 2002;14:416–442. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Yu-Chien WU, Andrew L, et al. Hybrid diffusion imaging. NeuroImage. 2007;36(3):617–629. doi: 10.1016/j.neuroimage.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Johnson B, et al. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010;204(1):57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Xu S, Proctor J, et al. Diffusion Kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59:467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou KH, Greve DN, et al. Reproducibility of functional MR imaging: preliminary results of prospective multi-institutional study performed by Biomedical Informatics Research Network. Radiology. 2005;237(3):781–789. doi: 10.1148/radiol.2373041630. [DOI] [PMC free article] [PubMed] [Google Scholar]


