Scheme 6a. Synthesis of the precusor (-)-33.
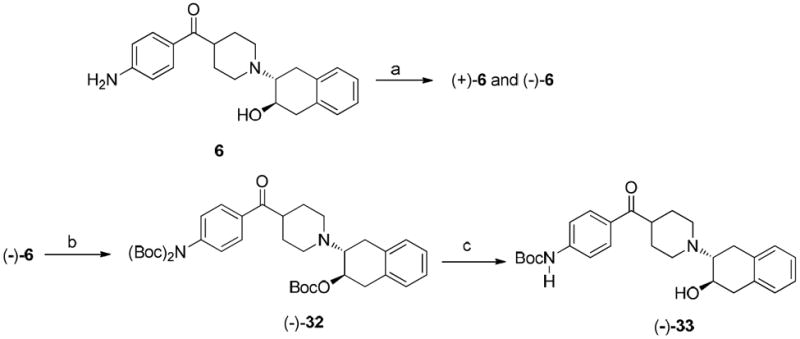
aReagents and conditions:
(a) Separation of enantiomers of 6 on HPLC: column: Chiralcel OD; mobile phase: 34% isopropanol in hexane; flow rate: 4.0 mL/min; (+)-enantiomer at 20.8 min and (-)-enantiomer at 33 min; (b) (Boc)2O, DMAP, Et3N, THF, rt., 1.5 h; (c) K2CO3, MeOH, reflux, overnight.
