Figure 1. Activation of G-protein-coupled receptors.
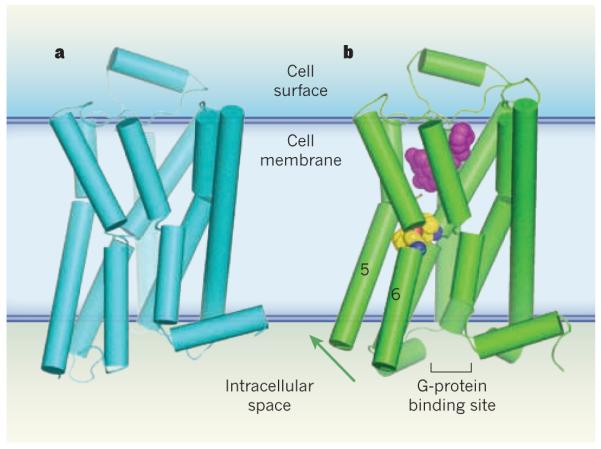
G-protein-coupled receptors (GPCRs) are a family of membrane-bound proteins. They activate G proteins when bound by agonist molecules, but are inactive in the presence of inverse agonists. a, The structure of the β-adrenergic receptor β2AR — a GPCR — is shown here in its inactive (R) state8. The receptor is bound to the inverse agonist carazolol (not shown). Cylinders indicate α-helices. b, Rasmussen et al.2 report the crystal structure of β2AR in complex with an agonist (purple) and with an antibody fragment (not shown), both of which stabilize the receptor’s active (R*) state. The antibody binds at the G-protein binding site, and acts as a substitute for β2AR’s G protein (Gs). The transition from the R to the R* state, induced by agonist binding, probably results from a contraction of the agonist binding site. The contraction promotes changes in the packing of amino-acid residues (yellow/blue/red spheres) between transmembrane helices 5 and 6. These changes coincide with rotation of parts of these helices (green arrow). Together, the conformational shifts create a binding site for the carboxy terminus of the G protein (or, in this case, the antibody fragment). This crystal structure2, along with structures and molecular simulations in two other papers3,4, suggest that both a G protein and an agonist must bind to GPCRs to stabilize the R* state.
