Abstract
Purpose
To estimate retinal ganglion cell (RGC) losses associated with the earliest development of visual field defects in glaucoma.
Design
Observational cohort study.
Participants
The study group included 53 eyes of 53 patients suspected of having glaucoma who were followed as part of the Diagnostic Innovations in Glaucoma (DIGS) study. These eyes had normal standard automated perimetry (SAP) visual fields at baseline and developed repeatable (3 consecutive) abnormal tests during a median follow-up of 6.7 years. An age-matched control group of 124 eyes of 124 healthy subjects recruited from the general population was included.
Methods
Estimates of RGC counts were obtained using a previously published model which combines estimates of RGC numbers from SAP sensitivity thresholds and retinal nerve fiber layer (RNFL) thickness measurements with spectral domain optical coherence tomography (SDOCT). For eyes converting to glaucoma, estimates of RGC counts were obtained at the time (within ± 3 months) of the first abnormal visual field, representing the time of earliest detection of visual field losses.
Main Outcome Measures
Estimates of RGC counts in eyes converting to glaucoma versus healthy eyes.
Results
The average RGC count estimate in the eyes with early visual field defects was 652057 ± 115829 cells, which was significantly lower than the average of 910584 ± 142412 cells found in healthy eyes (P<0.001). Compared to the average number of RGCs in the healthy group, glaucoma eyes had an average RGC loss of 28.4%, ranging from 6% to 57%, at the time of the earliest visual field defect on SAP. RGC counts performed significantly better than the SDOCT average RNFL thickness parameter in discriminating glaucomatous from healthy eyes with ROC curve areas of 0.95 ± 0.02 versus 0.88 ±0.03, respectively (P=0.001).
Conclusion
Glaucomatous eyes with the earliest detectable visual field loss on automated perimetry may already show substantial loss of retinal ganglion cells. Empirical estimates of RGC counts combining structural and functional tests agreed closely with previous histological reports on the number of RGCs associated with early visual fields defects on SAP.
INTRODUCTION
Glaucoma is a neurodegenerative disease associated with progressive loss of retinal ganglion cells (RGCs). The goal of glaucoma management is to slow down the rate of progressive neural losses in order to preserve visual function during the patient’s lifetime. Assessment of visual function in clinical practice is traditionally performed with standard automated perimetry (SAP). However, although SAP testing has been widely used for diagnosis, staging and monitoring the disease, it has become increasingly evident that a substantial number of RGCs may need to be lost before damage to SAP becomes statistically significant.1–10
In a study of cadaver eyes of glaucoma patients who had previously undergone SAP, Kerrigan-Baumrind and colleagues11 estimated that at least 25% to 35% of RGCs would need to be lost for statistically significant abnormalities to appear on automated perimetry. However, these estimates were based on a relatively small number of eyes and no follow-up data was available to determine precisely when visual field defects first occurred. Although direct RGC counting in vivo is not yet possible in humans, the use of empirical formulas derived from clinical structural and functional tests may give estimates of the number of RGCs which have been shown to correlate well with histologic counts in experimental glaucoma models.12, 13 In recent studies, we proposed a method for estimating the amount of RGC losses from a combination of retinal nerve fiber layer (RNFL) assessment with optical coherence tomography and SAP.14–16 The estimates of RGC counts performed significantly better than isolated structural and functional parameters for staging the disease and monitoring glaucomatous progression.
In the current study, we provided estimates of RGC losses associated with the earliest development of visual field defects in glaucoma. In order to assess RGC losses at this stage of the disease, a cohort of patients suspected of having glaucoma was followed until initial development of repeatable and statistically significant visual field defects on SAP. Using this approach, we were able to quantify the magnitude of estimated RGC losses associated with development of significant SAP abnormalities from the disease.
METHODS
This was an observational study. Participants from this study were included in two prospective longitudinal studies designed to evaluate optic nerve structure and visual function in glaucoma (the Diagnostic Innovations in Glaucoma Study [DIGS] and the African Descent and Glaucoma Evaluation Study [ADAGES] ). The 3-site ADAGES collaboration includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California-San Diego (UCSD) (data coordinating center), the New York Eye and Ear Infirmary and the Department of Ophthalmology, University of Alabama, Birmingham (UAB). Although the DIGS includes only patients recruited at UCSD, the protocols of the two studies are identical. The institutional review boards at all 3 sites approved the study methodology, which adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act. Methodological details have been described previously.17
At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, and automated perimetry using Swedish Interactive Threshold Algorithm (SITA Standard 24-2). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented with a best-corrected visual acuity less than 20/40, spherical refraction outside ± 5.0 diopters and/or cylinder correction outside 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
Participants
The study group consisted of 53 eyes of 53 patients suspected of having glaucoma who were followed as part of the DIGS/ADAGES cohort and developed repeatable abnormal visual fields during follow-up, i.e., converted to glaucoma. Initial diagnosis as glaucoma suspect was based on the presence of suspicious appearance of the optic disc or elevated (>21mmHg) intraocular pressure, but normal standard automated perimetry testing at baseline. Normal visual fields were defined based on mean deviation (MD) and pattern standard deviation (PSD) within 95% confidence limits and a Glaucoma Hemifield Test (GHT) within normal limits. These eyes had a median follow-up of 6.7 years (1st quartile: 4.4 years, 3rd quartile: 13.3 years) until the development of repeatable abnormal SAP defects. Repeatable abnormal SAP was defined based on the presence of a sequence of 3 consecutive abnormal SAPs with PSD with P<5% or Glaucoma Hemifield Test outside normal limits. Imaging assessment of the RNFL with spectral domain optical coherence tomography (SDOCT) was performed at the time (within ± 3 months) of the first visual field of the sequence of 3 repeatable abnormal fields. This was performed in order to calculate estimates of RGC counts (see below) at the time of detection of the earliest visual field defect on SAP.
An age-matched control group was included in the study consisting of 124 eyes from 124 healthy participants. These subjects were recruited from the general population and were required to have a normal ophthalmologic examination and IOP below 22mmHg in both eyes, but results of visual field tests and SDOCT were not used as inclusion or exclusion criteria. Healthy eyes were chosen as control group because we were interested in evaluating the amount of RGC loss associated with early visual field defects compared to normal expected age-matched RGC counts. Although a group of glaucoma suspects who did not develop visual field loss could be initially thought as a control group, these eyes could have sustained structural damage before functional losses and, therefore, would not constitute a suitable control group for the purposes of this study.
Visual Field Testing
All patients underwent SAP testing using SITA-standard 24-2 strategy during follow-up. All visual fields were evaluated by the UCSD Visual Field Assessment Center (VisFACT).18 Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors were excluded. Visual fields exhibiting a learning effect (i.e., initial tests showing consistent improvement on visual field indexes) were also excluded. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention. The VisFACT requested repeats of unreliable visual field test results, and these were obtained whenever possible.
Spectral-Domain OCT
The Cirrus HDOCT (software version 5.2, Carl Zeiss Meditec Inc., Dublin) was used to acquire RNFL measurements in the study. It uses a superluminescent diode scan with a center wavelength of 840 nm and an acquisition rate of 27 000 A-scans per second at an axial resolution of 5 µm. The protocol used for RNFL thickness evaluation was the optic disc cube. This protocol is based on a 3-dimensional scan of a 6×6 mm2 area centered on the optic disc where information from a 1024 (depth) × 200 × 200-point parallelepiped is collected. Then, a 3.46-mm diameter circular scan (10870µm length) is automatically placed around the optic disc, and the information about parapapillary RNFL thickness is obtained. Because information from the whole region is obtained, it is possible to modify the position of the scan after the exam is taken. To be included, all images were reviewed for non-centered scans and had to have signal strength >6, absence of movement artifacts, and good centering on the optic disc. For estimation of overall RGC counts, we used the parameter average RNFL thickness (360° measure around the optic disc). For estimation of RGC counts on each hemiretina, we calculated the average RNFL thickness at each semicircle of 180° around the optic disc.
Estimates of Retinal Ganglion Cell Counts
The estimates of RGC counts were obtained according to the model developed by Medeiros et al14, 15 based on empirical formulas derived by Harwerth et al for estimating ganglion cell counts from SAP and OCT. The model uses information from structural and functional tests to derive a final estimate of the RGC count in a particular eye. The details of the model and the empirical formulas used to derive RGC counts have been described in detail in previous publications.14, 15 The initial step of the model consists in translating SAP sensitivity values into RGC counts using empirical formulas derived by experimental research in monkeys and subsequently translated to normal and glaucomatous human eyes.4, 13 The following formulas were used to estimate the number of RGC somas in an area of the retina corresponding to a specific SAP test field location at eccentricity ec with sensitivity s in dB:
m = [0.054*(ec*1.32)] + 0.9
b = [−1.5*(ec*1.32)] − 14.8
gc = {[(s−1) −b]/m} + 4.7
SAPrgc = Σ 10^(gc*0.1)
In the above formulas, m and b represent the slope and intercept, respectively, of the linear function relating ganglion cell quantity (gc) in dB to the visual field sensitivity (s) in dB at a given eccentricity. To account for the total number of ganglion cells in an area of the retina, the cell density derived from each perimetry measurement was considered to be uniform over an area of retina corresponding to an area of 6×6 degrees of visual space that separates test locations in SAP. By applying the above formulas, a SAP-derived estimate of the total number of RGCs (SAPrgc) was obtained by adding the estimates from all locations in the visual field. The structural part of the model consisted in estimating the number of RGC axons from RNFL thickness measurements obtained by optical coherence tomography. The model took into account the effect of aging in the axonal density and the effect of disease severity on the relationship between the neuronal and non-neuronal components of the RNFL thickness estimates obtained by OCT. To derive the total number of RGC axons from the global RNFL thickness measurement obtained by OCT (OCTrgc), we applied the following formulas:
d = (−0.007*age) + 1.4
c = (−0.26*MD) + 0.12
a = average RNFL thickness * 10870*d
OCTrgc = 10^[(log(a)*10 −c)*0.1]
In the above formulas, d corresponds to the axonal density (axons/µm2) and c is a correction factor for the severity of disease to take into account remodeling of the RNFL axonal and non-axonal composition. These calculations provide an estimate of the number of RGCs from two sources, one functional and one structural. A combined calculation of RGC counts was performed according to the following formula:
RGC count = (1 + MD/30)*OCTrgc + (−MD/30)*SAPrgc
The rationale for using a weighting system for deriving the final RGC count is described by Medeiros et al,14–16 but in essence it relies on the fact that the accuracies of clinical perimetry and imaging tests are inversely related to disease severity.
RGC counts were also obtained separately for each hemifield of the retina, using corresponding visual field sensitivities and retinal nerve fiber layer thickness measurements.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for normally distributed variables, and median, first quartile and third quartile values for non-normally distributed variables. Student t tests or Mann-Whitney U tests were used to evaluate demographic and clinical differences between glaucoma and control subjects in each one of the analyses.
The performance of the RGC counts to discriminate glaucoma eyes with early visual field defects from healthy eyes was compared to that of standard SDOCT parameters. No comparison was performed against visual field parameters as these were used in the definition of the glaucoma group. Receiver operating characteristic (ROC) curves were built, and the area under the ROC curve was used to summarize the diagnostic accuracy for each parameter. An ROC curve area equal to 1 represents perfect discrimination, whereas an area of 0.5 represents chance discrimination. ROC curve areas and 95% confidence intervals were obtained for each parameter after adjusting for age, using a previously described method.19, 20 Evaluation of diagnostic accuracy was also performed using likelihood ratios (LR). LR is defined as the probability of a given test result in those with disease divided by the probability of the same test result in those without disease.21, 22 Once determined, an LR can be directly incorporated into the calculation of posttest probability of disease by using a formulation of the Bayes’ theorem.23 The LR for a given test result indicates how much that result will raise or decrease the pretest odds of disease. Application of LRs in the interpretation of results of imaging instruments for glaucoma diagnosis has been detailed previously.24, 25 A value of 1 means that the test provides no addition information, and ratios higher or lower than 1 increase or decrease the likelihood of disease, respectively.
All statistical analyses were performed with commercially available software (Stata version 12; StataCorp, College Station, TX). The alpha level (type I error) was set at 0.05.
RESULTS
There were 53 eyes of 53 subjects who developed visual field loss during follow-up and were included in the glaucoma group. At the baseline visit, average MD and PSD for these eyes were −0.98 ± 1.39 dB and 1.96 ± 0.56 dB, respectively. Corresponding values were −2.17 ± 1.34dB and 2.48 ± 0.44 dB, respectively, at the time of the first abnormal visual field of the conversion sequence, i.e., at the time of estimation of RGC counts. The average age at the time of conversion was 69 ± 12 years. This group was compared to 124 eyes of 124 healthy subjects with average age of 66 ± 11 years. There was no statistically significant difference in mean age between the two groups (P = 0.07). Average MD and PSD values for the healthy eyes were 0.11 ± 1.23 dB and 1.67 ± 0.59 dB, respectively. Table 1 summarizes clinical and demographic parameters in the glaucoma and control groups.
Table 1.
Clinical and Demographic Variables in the Glaucoma and Healthy Groups.
| Glaucoma (n = 53) |
Healthy (n = 124) |
P | |
|---|---|---|---|
| Age | 69 ± 12 | 66 ± 11 | 0.07 |
| Race | |||
| Caucasians | 37 | 94 | 0.41 |
| African-Americans | 16 | 30 | |
| Gender, female (%) | 33 (62) | 85 (69) | 0.42 |
| MD* | −2.17 ± 1.34 | 0.11 ± 1.23 | <0.001 |
| PSD* | 2.48 ± 0.44 | 1.67 ± 0.59 | <0.001 |
| Average RNFL thickness | 76.0 ± 9.9 | 91.6 ± 8.9 | <0.001 |
| Estimated RGC count | 652057 ± 115829 | 910584 ± 142412 | <0.001 |
MD and PSD for glaucoma eyes correspond to the values obtained from the first abnormal visual field of the conversion sequence. Values correspond to mean ± standard deviation, unless specified otherwise.
MD: Mean Deviation; PSD: Pattern Standard Deviation. RNFL: Retinal nerve fiber layer; RGC: Retinal ganglion cell.
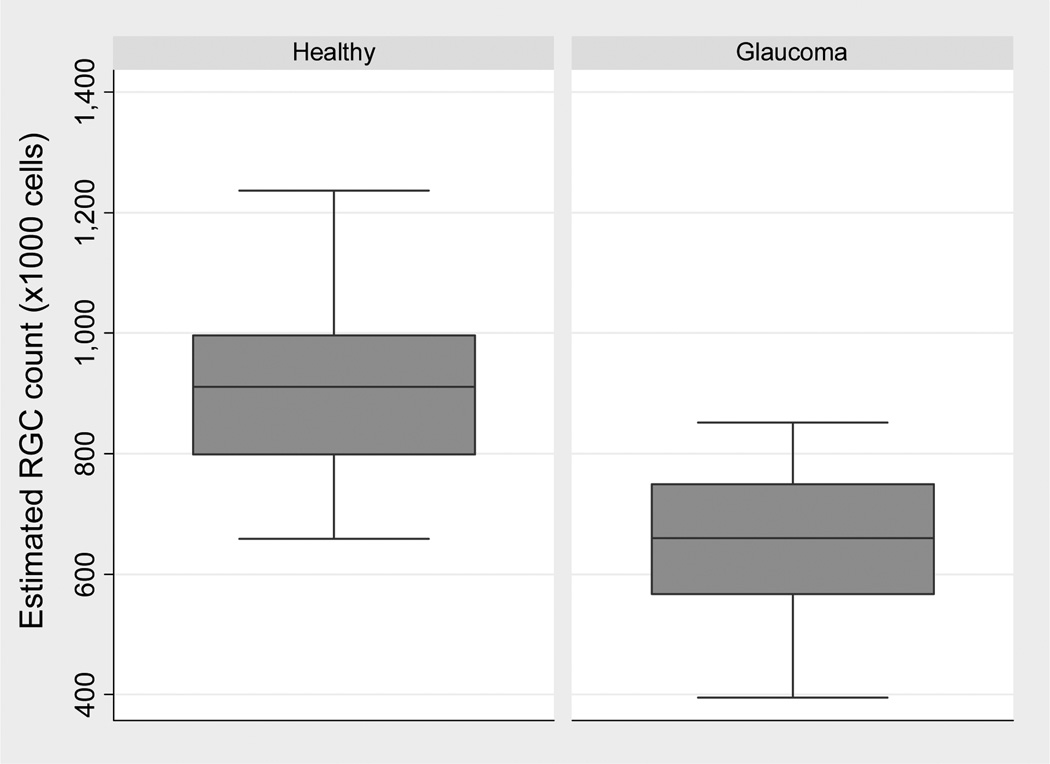
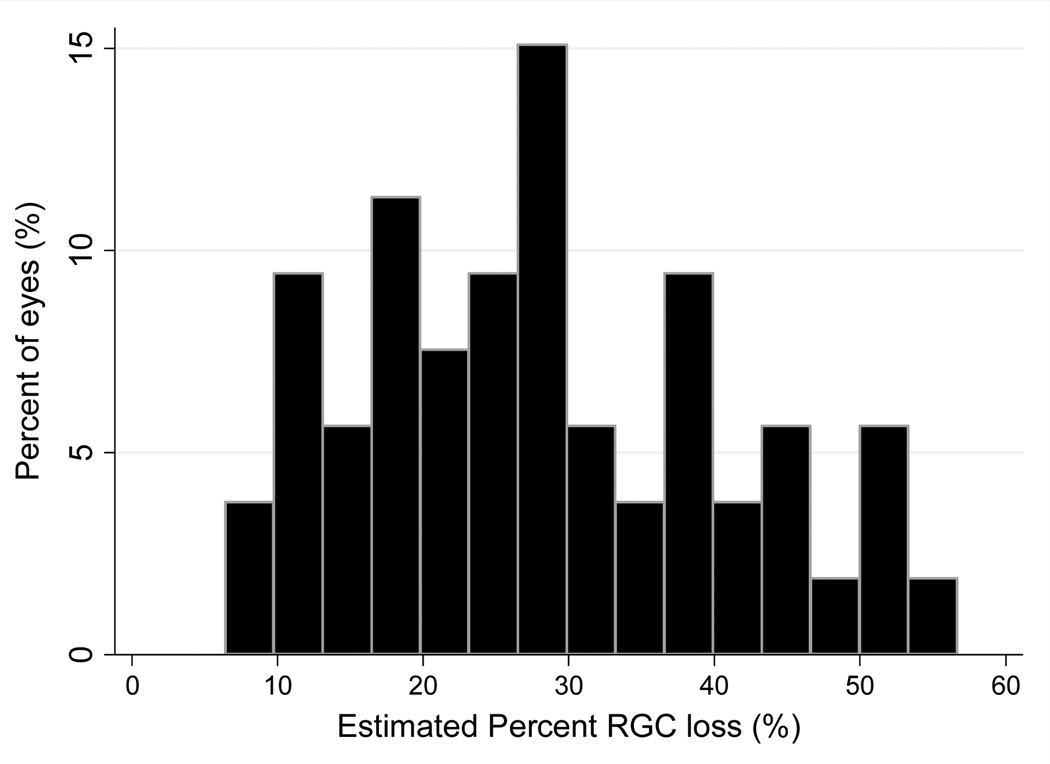
The average RGC count estimate in the eyes with early visual field defects was 652057 ± 115829 cells, which was significantly lower than the average of 910584 ± 142412 cells found in healthy eyes (P<0.001). Figure 1 illustrates the distribution of RGC estimates in the glaucoma and control groups. Compared to the average number of RGCs in the healthy group, glaucoma eyes had an average RGC loss of 28.4% (95% CI: 24.9% to 31.9%), ranging from 6% to 57%. Figure 2 illustrates the distribution of percent RGC losses in the glaucoma group.
Figure 1.
Boxplots illustrating the distribution of estimated retinal ganglion cell (RGC) counts in glaucoma eyes with early visual field defects and control healthy eyes.
Figure 2.
Distribution of estimated percent losses of retinal ganglion cells (RGCs) in the glaucoma eyes with early visual field defects
Twenty-two of the 53 (42%) glaucoma eyes developed superior visual field defects, 14 (26%) developed inferior defects and in 17 eyes (32%) defects were seen both superiorly and inferiorly. For the 22 eyes with superior visual field defects, RGC counts corresponding to the inferior hemiretina were significantly lower than those from the superior hemiretina (283341 ± 55526 vs. 340931 ± 63888, respectively; P<0.001). For the 14 eyes with inferior defects, RGC counts from the superior hemiretina were significantly lower than those from the inferior hemiretina (303964 ± 56160 vs. 360191 ± 75103, respectively; P <0.001). For the 17 eyes with defects both superiorly and inferiorly, there was no statistically significant difference between RGC counts in the superior and inferior hemiretinas (343849 ± 58424 vs. 329762 ± 56306, respectively; P=0.12). For the 124 healthy eyes, there was no significant difference between RGC counts in the superior and inferior hemiretinas (459557 ± 75292 vs. 451447 ± 76208, respectively; P = 0.052).
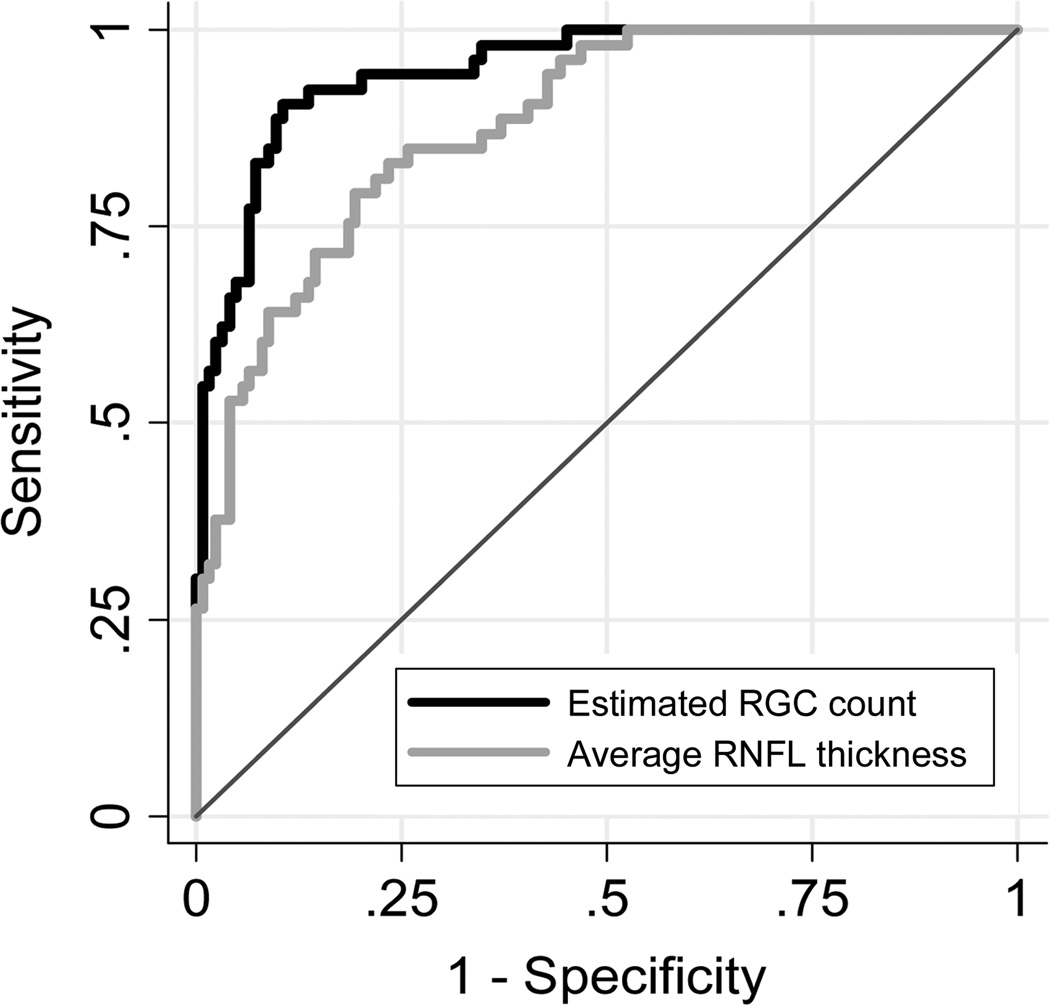
RGC counts performed significantly better than the SDOCT average RNFL thickness parameter in discriminating glaucomatous from healthy eyes with ROC curve areas of 0.95 ± 0.02 versus 0.88 ±0.03, respectively (P=0.001) (Figure 3). For 95% specificity, RGC counts had sensitivity of 68% for detection of early glaucomatous damage with positive LR of 13.6, whereas SDOCT average RNFL thickness had sensitivity of 53% with a positive LR of 10.6. For 90% specificity, sensitivity of RGC counts increased to 89% versus 64% for SDOCT average RNFL thickness.
Figure 3.
Receiver operating characteristic curves for discriminating glaucomatous eyes with early visual field defects from healthy eyes for the estimated retinal ganglion cell (RGC) counts and the average retinal nerve fiber layer (RNFL) thickness parameter.
Case Examples
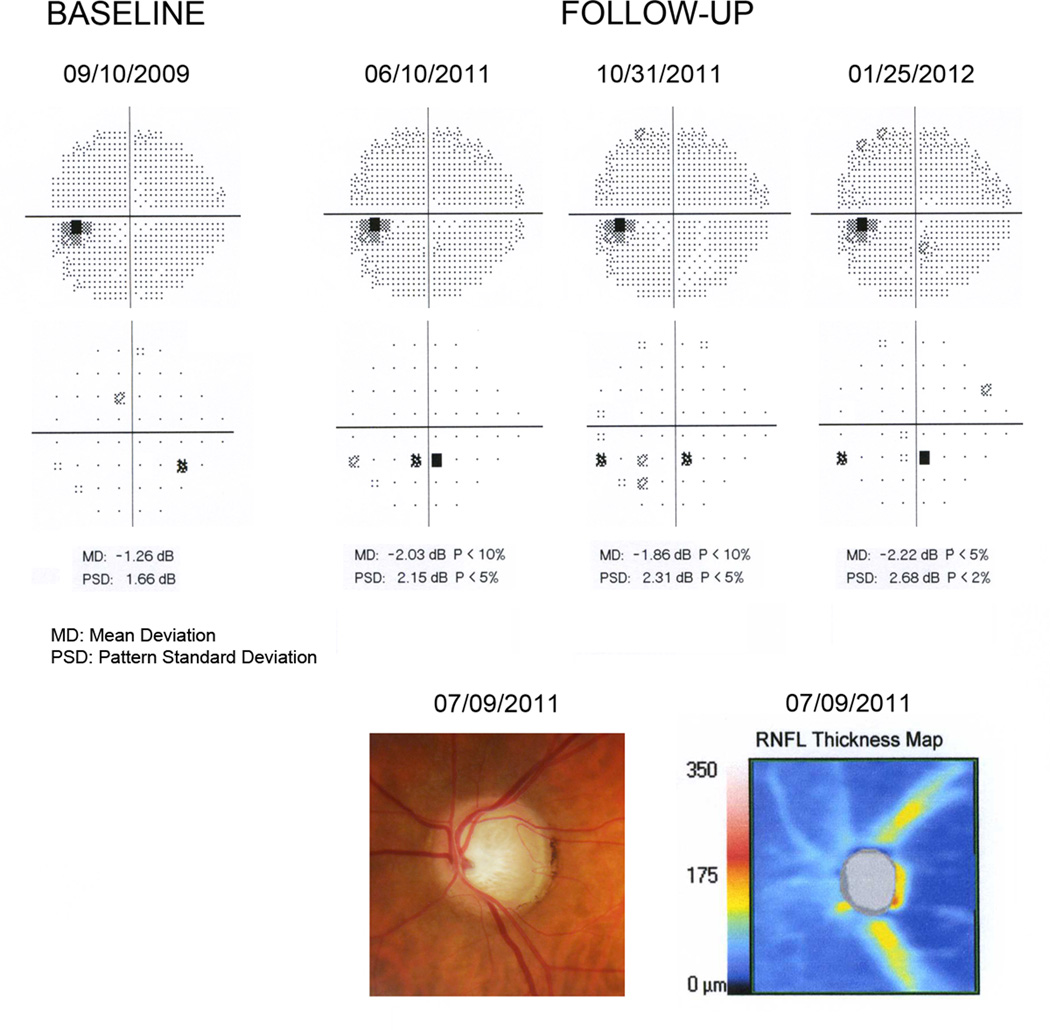
Figure 4 illustrates an eye that had an estimated RGC count of 520950 cells at the time of development of the initial visual field defect on SAP, corresponding to 43% RGC loss compared to the healthy group. The defect was confirmed on subsequent tests based on the criterion of 3 consecutive abnormal fields with PSD with P<5%. The optic disc photograph shows extensive neuroretinal rim loss in agreement with the RNFL loss assessed by SDOCT, which showed average RNFL thickness of 58µm. Despite the extensive RGC loss, the visual field defect on the pattern deviation plot was apparently small with only an inferior cluster of abnormal points, although there was evidence of diffuse loss of sensitivity as indicated by the MD of −2.14dB (P<5%).
Figure 4.
Grayscale and pattern deviation plots for the visual fields for one of the glaucomatous eyes in the study. The normal baseline visual field is shown along with the 3 consecutive abnormal visual fields during follow-up. The remaining normal visual fields between baseline and first abnormal field were omitted. Estimates of retinal ganglion cell (RGC) counts were calculated using data from the first abnormal visual field (6/10/2011) and from the spectral domain optical coherence tomography (07/09/2011). The eye had an estimated RGC count of 520950 cells at the time of development of the initial visual field defect on standard automated perimetry (SAP), corresponding to 43% RGC loss compared to the healthy group. This is in agreement with extensive neuroretinal rim loss seen on the optic disc photograph.
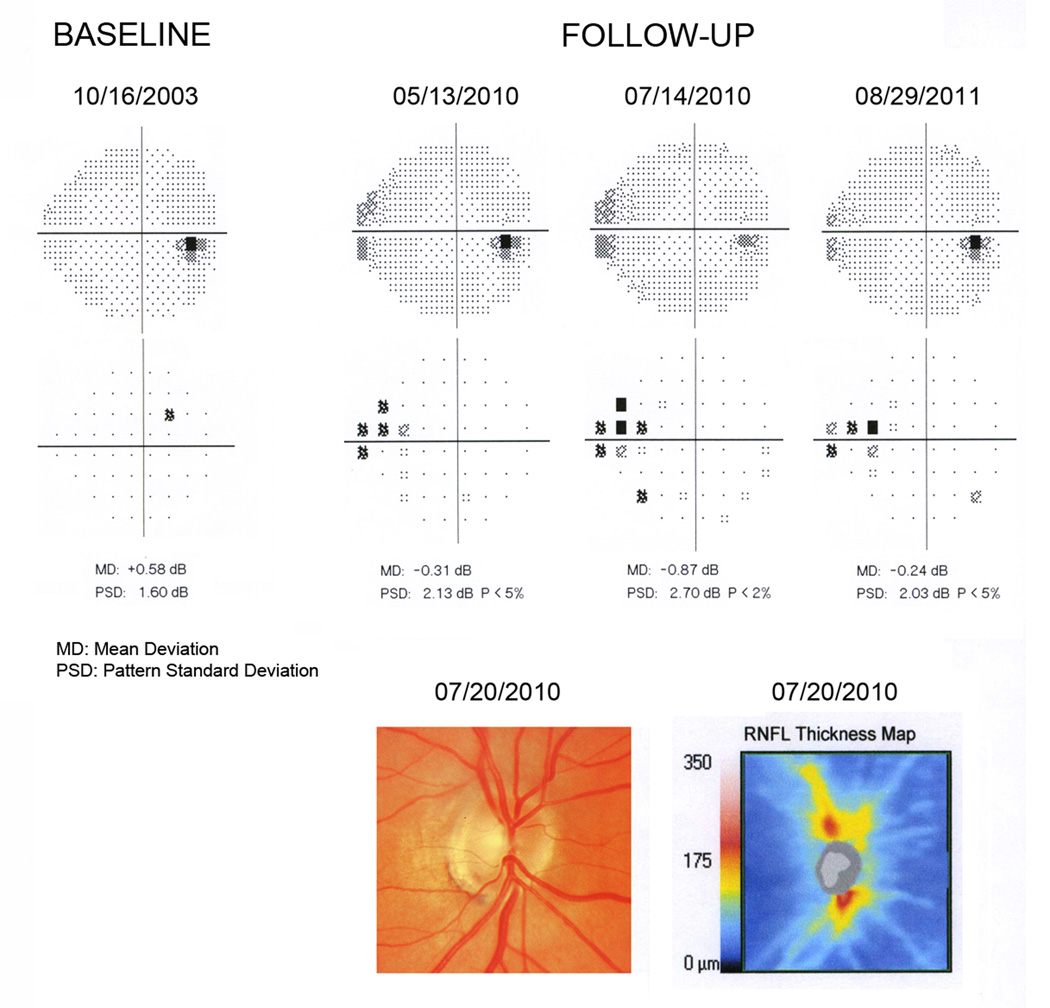
Figure 5 shows an eye with an estimated RGC count of 800369 at the time of development of the initial visual field defect, which corresponded to 12% RGC loss compared to the healthy group. The optic disc photograph shows inferior neuroretinal rim thinning in agreement with inferior RNFL loss detected by SDOCT. Average RNFL thickness was 80µm. Visual fields show a more localized defect compared to the eye shown on Figure 4, with an abnormal PSD but MD within normal limits.
Figure 5.
Grayscale and pattern deviation plots for the visual fields for one of the glaucomatous eyes in the study. The normal baseline visual field is shown along with the 3 consecutive abnormal visual fields during follow-up. The remaining normal visual fields between baseline and first abnormal field were omitted. Estimates of retinal ganglion cell (RGC) counts were calculated using data from the first abnormal visual field (5/13/2010) and from the spectral domain optical coherence tomography (07/20/2010). The eye had an estimated RGC count of 800369 at the time of development of the initial visual field defect, which corresponded to 12% RGC loss compared to the healthy group. The optic disc photograph shows inferior neuroretinal rim thinning in agreement with inferior retinal nerve fiber layer (RNFL) loss detected by spectral domain optical coherence tomography.
DISCUSSION
In the current study, we used empirical formulas to estimate RGC counts in suspect eyes converting to glaucoma at the time of the earliest development of visual field defects in comparison to a group of healthy eyes. Our results suggest that a substantial number of RGCs may be lost by the time early visual field changes are detectable on standard automated perimetry. Eyes with early visual field defects on our study had an average estimated RGC count of 652057 cells versus 910584 cells in the healthy group with similar age. This translates into an estimated average RGC loss of 28.4% associated with early visual field defects. This number is remarkably similar to that found by Kerrigan-Baumrind et al11 in histologic studies of human eyes. The authors studied 17 post-mortem eyes of 13 subjects with well-documented history of glaucoma and compared the histological RGC counts to those obtained from 17 post-mortem eyes of 17 age-matched healthy controls. They found that the average RGC loss in eyes with PSD or corrected pattern standard deviation (CPSD) with P less than 5% was 27.3%. These observations are also in agreement with other qualitative and quantitative clinical studies suggesting that substantial damage can occur to the optic nerve and RNFL before visual field defects are detectable on standard automated perimetry. 2–10
In order to be able to estimate RGC losses associated with the earliest detectable visual field losses on SAP, we longitudinally followed a cohort of glaucoma suspects over time until they showed evidence of repeatable visual field defects. The criteria used to define visual field losses were those applied by the Ocular Hypertension Treatment Study (OHTS)5, 26 and widely used in clinical practice, requiring confirmation of abnormalities in 3 consecutive visual fields. This greatly decreases the chance that the abnormalities seen on perimetry may represent just variability rather than true defects. The calculations of estimated RGC counts were performed at the time corresponding to the first abnormal visual field and, therefore, would reflect the amount of neural damage seen at the time of the first abnormality detected by perimetry in clinical practice. As the eyes were observed during the transition period from normal to abnormal visual fields, this design provides a more robust determination of the point of earliest development of field losses than cross-sectional investigations.
Our method of estimating RGC counts relies on calculations of the number of RGCs estimated from data acquired by both SAP and OCT RNFL thickness evaluation. Empirical formulas for RGC count estimation from SAP and OCT were developed by Harwerth and colleagues.13 Using normal monkeys and monkeys with laser-induced experimental glaucoma, they showed that SAP sensitivity values can provide good estimates of the amount of histologically-measured RGC counts in the retina. These estimates agreed closely with those obtained from OCT RNFL thickness data. They showed a strong linear relationship between the number of RGC somas and axons obtained from functional and structural measures, respectively, when retinal eccentricity and appropriate measurement scales for neural and sensitivity losses were used. The linear relationship suggests that the lack of sensitivity of SAP for detection of early glaucomatous damage is most likely not the result of true structural changes occurring in the absence of functional losses, but is rather related to the logarithmic scale used for SAP sensitivity measurements, as well as the magnitude of change required to reach statistically significant levels of abnormality.3, 27 The logarithmic scale compresses the range of losses in early stages of the disease while expanding the range in later stages. These findings could suggest that a simple linearization of SAP data could improve detection of early damage. However, although linearization of SAP measurements improves the structure and function relationship on population data, it generally does not improve the sensitivity to early losses in an individual patient.14 As SAP sensitivity thresholds are originally acquired using staircase procedures in dB units, the compression of the range of losses in early stages of the disease caused by the logarithmic scale will still be present. Because of the weighting system for obtaining final RGC counts, our method relies much more heavily on OCT data than SAP for estimation of early neural losses. It should be noted, however, that there was still a significant contribution from SAP data in the RGC count estimates. This can be seen from the fact that estimated RGC counts performed significantly better than SDOCT average thickness in discriminating glaucoma from healthy eyes, with ROC curve areas of 0.95 versus 0.88, respectively, and higher sensitivities at fixed specificities. These results suggest that our proposed method for combining structural and functional data may perform better than isolated structural or functional tests for detection of early glaucomatous damage. In addition, calculation of LRs for estimated RGC counts showed large effects on the probability of disease, giving further indication of the utility of this approach in clinical practice.22
In a previous investigation, we demonstrated that estimates of RGC counts obtained by the same method applied in the current study were able to detect preperimetric glaucomatous damage, i.e., before the development of visual field defects.14 Eyes with preperimetric damage had documented evidence of progressive glaucomatous damage on optic disc stereophotographs. These eyes had an average estimated loss of 17% of RGCs from age-expected RGC numbers. As expected, the average estimated percent RGC loss in eyes with visual field defects found in our study was greater than that of eyes with preperimetric damage. For eyes with moderate perimetric damage (average MD of −8.2dB), the previously estimated average RGC loss was 52%, whereas for eyes with advanced damage (average MD of −17.4) it was 75%.14 In another study, we showed that RGC counts performed better than isolated structural or functional parameters for detecting progressive glaucomatous damage over time.15 The results of the present investigation combined with our previous studies suggest that our proposed method for estimating RGC counts could be a useful tool for detection of glaucomatous damage throughout the spectrum of the disease.
Early detection and quantification of RGC losses in glaucoma may carry significant implications for the patient, even if they are not yet associated with detectable SAP losses. If substantial damage has already occurred by the time the disease is diagnosed, a relatively smaller number of RGCs will need to be lost before the number of cells reaches critical levels associated with disability from the disease. Although such critical levels cannot be presently ascertained for particular individuals, recent evidence suggests that decrease in vision-related quality of life from glaucoma is seen sooner than previously anticipated.28 Therefore, if treatment is initiated late in the course of the disease, a much slower rate of change will have to be achieved in order to prevent development of functional impairment than what would be necessary if treatment had been started earlier. Although it is generally possible to slow down the rate of disease progression and keep patients close to stability even if they have moderate or advanced damage29, this usually requires more aggressive interventions with larger potential for side effects, compared to what would be necessary if treatment had been started at an earlier stage. In 20% of the eyes with early visual field defects included in our study, the estimated RGC losses amounted to over 40%, with an average RGC count of only 480216 cells by the time the earliest visual field defect was detected on SAP. If we assume that functional impairment would occur with moderate to severe visual field damage, i.e., with an RGC count of approximately 300,000 RGCs based on previous data14, these eyes would need to lose an additional number of only 180,000 RGCs to go from early visual field defect to functional impairment, a much lower number than what was lost before development of early field defects. It is important to emphasize, however, that the results of our study should not necessarily be taken as evidence that patients with optic nerve damage but no apparent visual field loss need to be treated. Although early treatment may be beneficial in many situations, decisions about treatment need to take into account several considerations such as rate of disease progression, patient’s life expectancy, risks of treatment and patient’s expectations about the disease and its treatment.
There was a large variation of the estimates of RGC losses in eyes with early visual field defects. This could be due to several reasons, such as variability of the tests used to estimate RGC counts, as well as the characteristics of the visual field defects detected by SAP. As the OHTS criterion used to detect visual field defects is essentially based on localized visual field losses or asymmetric damage on the GHT, it can potentially miss eyes with diffuse losses of sensitivity caused by diffuse neural losses in glaucoma. In fact, the eye illustrated on Figure 4 shows extensive neural damage with an estimated average RGC loss of 43%, but only a relatively small localized visual field defect. There was, however, evidence of diffuse visual field losses as measured by the MD of −2.14dB (P<5%). On the other hand, the eye shown on Figure 5 shows a more localized visual field defect without evidence of diffuse losses and the estimated RGC damage was only 12%. This is in agreement with previous studies suggesting that pattern deviation analysis of SAP data may significantly underdiagnose glaucomatous eyes with diffuse losses of sensitivity.30 Detection of eyes with diffuse loss of sensitivity is difficult due to the confounding effects of media opacities. This finding highlights the need for a combined approach of structural and functional evaluation for detection of eyes with different patterns of glaucomatous damage.
Our study has limitations. We used empirically-derived formulas to estimate the number of RGCs from SAP and OCT data and our estimates of RGC counts were not based on direct histologic RGC counts in humans. The empirical formulas derived by Harwerth and colleagues13 have been validated on histological studies in monkeys which have a visual system almost indistinguishable to that of humans. The relationship between predicted RGC counts and histologically-measured RGC numbers had an R2 of 0.9, indicating an almost perfect predictive value. They have also been applied to multiple external cohorts in humans.13 It should be noted that there have been little to no histological validations of measures such as ganglion cell complex or even RNFL thickness as performed by OCT instruments. However, this carries little significance as long as one shows that these measurements have clinical relevance. Furthermore, our estimates agreed remarkably well with histological studies in human glaucomatous eyes, as discussed above. Another limitation of our study is that we did not have longitudinal follow-up with SDOCT over the same time course as SAP, which prevented us from obtaining estimates of RGC counts throughout the follow-up in glaucoma suspect eyes. It should be noted, however, that even if longitudinal data on RGC counts were available it would be impossible to determine the true individual amount of RGC losses from the disease as we currently have no way of determining when the glaucomatous damage started to occur. The design of our study addressed this limitation in the best possible way by comparing the estimates to an age-matched healthy population. We did not have follow-up data on the healthy eyes included in the control group, which would have allowed us to estimate age-related RGC losses and potentially better estimation of RGC losses in individual eyes. However, the healthy eyes had similar age than those in the glaucoma group and, therefore, we still expect that our overall conclusions with regard to the average number of RGC losses would be correct.
In conclusion, glaucomatous eyes with the earliest detectable visual field losses on automated perimetry already show substantial losses of estimated RGC counts. Our proposed method to estimate RGC counts based on a combination of structural and functional tests may allow detection and quantification of neural damage in these eyes with better diagnostic accuracy compared to standard parameters from imaging instruments.
Acknowledgments
Supported in part by NIH/NEI grants EY021818 (FAM), EY11008 (LMZ), EY14267 (LMZ), EY13959 (CAG), unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at University of California San Diego, the Eyesight Foundation, Grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen Financial Disclosures: Research support from Carl-Zeiss Meditec (FAM, LMZ, CAG, JML, RNW). Research support from Heidelberg Engineering (FAM, LMZ, RNW, JML). Consultant to Carl-Zeiss Meditec, Inc. (RNW).
REFERENCES
- 1.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127:1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwerth RS, Carter-Dawson L, Smith EL, III, et al. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–3160. doi: 10.1167/iovs.04-0227. [DOI] [PubMed] [Google Scholar]
- 5.The Ocular Hypertension Treatment. Kass MA, Heuer DK, Higginbotham EJ, et al. Study Group The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 6.European Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–2910. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 9.Leung CK, Cheung CY, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010;51:217–222. doi: 10.1167/iovs.09-3468. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros FA, Alencar LM, Zangwill LM, et al. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116:1125–1133. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- 12.Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL., III The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–773. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 13.Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29:249–271. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros FA, Lisboa R, Weinreb RN, et al. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol. 2012;130:1107–1116. doi: 10.1001/archophthalmol.2012.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medeiros FA, Zangwill LM, Anderson DR, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. doi: 10.1016/j.ajo.2012.04.022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros FA, Zangwill LM, Bowd C, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.12-10345. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sample PA, Girkin CA, Zangwill LM, et al. ADAGES Study Group. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ADAGES Group. Racette L, Liebmann JM, Girkin CA, et al. African Descent Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128:551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou XH, Obuchowksi NA, McClish DK. Statistical Methods in Diagnostic Medicine. New York: Wiley-Interscience; 2002. pp. 274–306. [Google Scholar]
- 20.Alonzo TA, Pepe MS. Distribution-free ROC analysis using binary regression techniques. Biostatistics. 2002;3:421–432. doi: 10.1093/biostatistics/3.3.421. [DOI] [PubMed] [Google Scholar]
- 21.Radack KL, Rouan G, Hedges J. The likelihood ratio. An improved measure for reporting and evaluating diagnostic test results. Arch Pathol Lab Med. 1986;110:689–693. [PubMed] [Google Scholar]
- 22.Evidence-Based Medicine Working Group. Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 23.Fagan TJ. Letter: Nomogram for Bayes theorem. N Engl J Med. 1975;293:257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 24.Medeiros FA, Zangwill LM, Bowd C, et al. Use of progressive glaucomatous optic disk change as the reference standard for evaluation of diagnostic tests in glaucoma. Am J Ophthalmol. 2005;139:1010–1018. doi: 10.1016/j.ajo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FA, Zangwill LM, Bowd C, Weinreb RN. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and Stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–837. doi: 10.1001/archopht.122.6.827. [DOI] [PubMed] [Google Scholar]
- 26.Ocular Hypertension Treatment Study Group. Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 27.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41:1774–1782. [PubMed] [Google Scholar]
- 28.McKean-Cowdin R, Varma R, Wu J, et al. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–1023. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 30.Ocular Hypertension Treatment Study Group. Artes PH, Chauhan BC, Keltner JL, et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:1528–1532. doi: 10.1001/archophthalmol.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]