Figure 1.
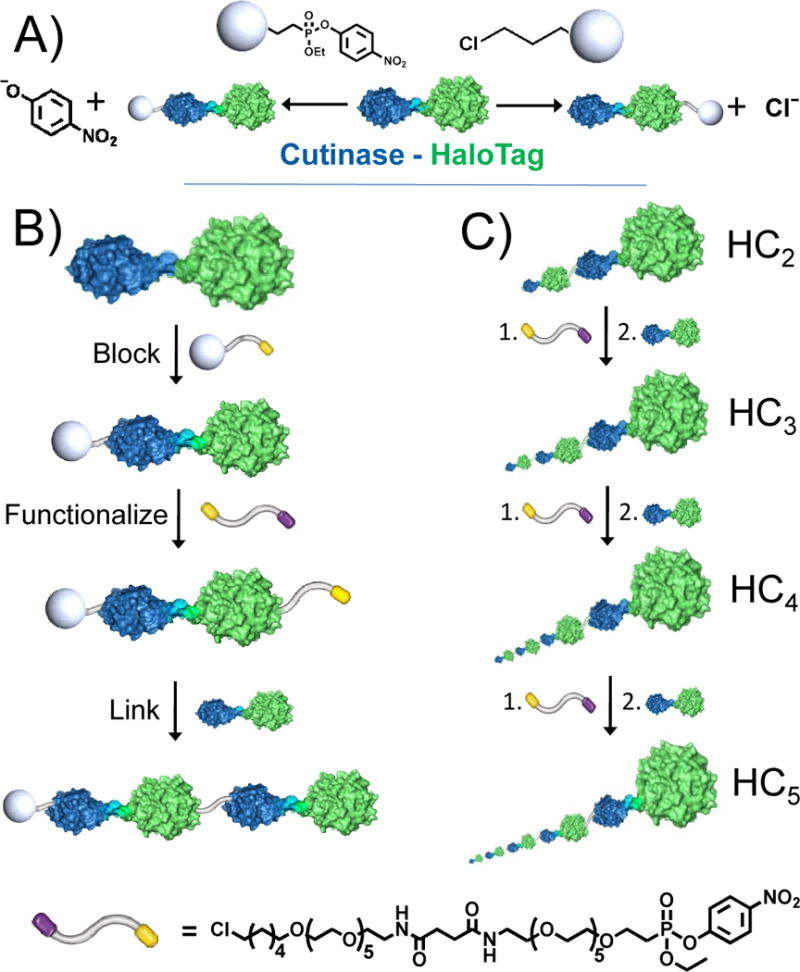
This paper describes an approach for synthesizing megamolecules through a step-wise joining of a fusion protein prepared from cutinase (blue) and HaloTag (green) and of a bifunctional linker terminated in irreversible inhibitors for the proteins. A) The fusion protein (HC) can be blocked at the cutinase or HaloTag domain by reaction with a phosphonate or hexylchloride group, respectively. B) A dimeric structure can be assembled by allowing the fusion protein to react with the linker followed by another equivalent of HC protein. C) Repetition of this cycle gives oligomeric forms of the HC protein. The bifunctional linker is terminated in p-nitrophenylphosphonate (yellow) and an alkylchloride (purple) group (bottom). The proteins in panels a and b are shown to scale and those in panel c are shown in perspective with respect to the plane of the page.
