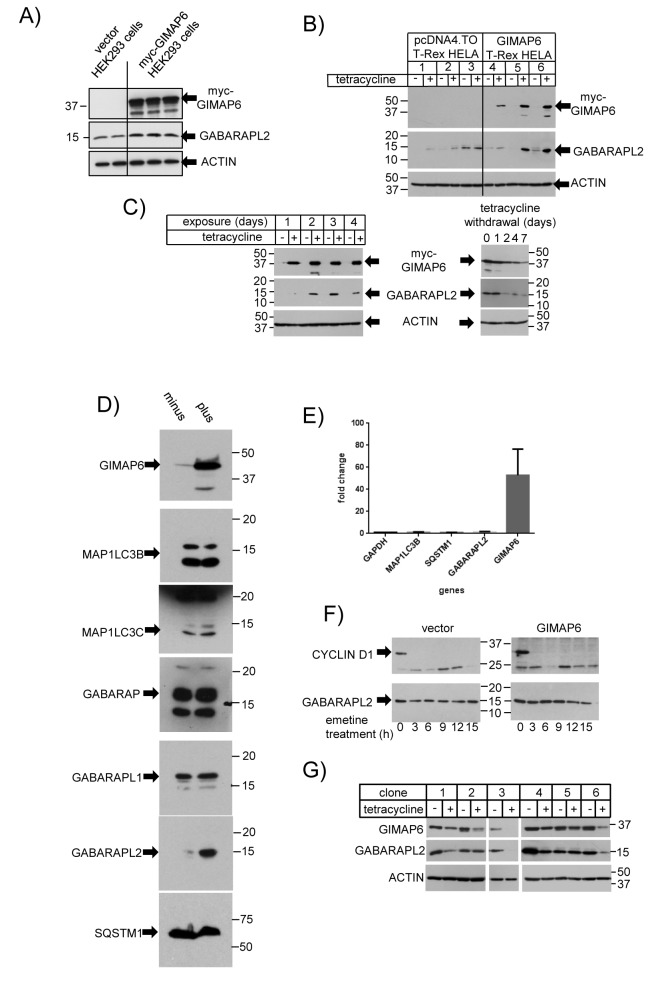
Figure 4. GIMAP6 over-expression leads to the induction of GABARAPL2.
A) Cells lysates were prepared from three myc-GIMAP6 HEK293 cell lines (lanes 3-5) or two cell lines carrying the corresponding vector (lanes 1-2) and expression of GIMAP6, GABARAPL2, and β-actin analysed by Western blotting as indicated. B) Three T-RexTM-HeLa cell lines carrying plasmid pcDNA4.TO (lanes 1-3) or three myc-GIMAP6 T-Rex HeLa cell lines (lanes 4-6) were grown in the presence or absence of 2 µg/ml tetracycline for four days. Cells lysates were prepared and analysed for myc-GIMAP6, GABARAPL2 or β-actin expression, as indicated, by Western blotting. C) A myc-GIMAP6 T-Rex Hela cell line was grown for various times in the presence or absence of tetracycline. After 4 days, some dishes of cells that had been grown in the presence of tetracycline were extensively washed and maintained in the absence of tetracycline for further time intervals. At each time-point, cell lysates were prepared and analysed for GIMAP6, GABARAPL2 or β-actin expression (with primary antibodies: rat anti-GIMAP6 monoclonal antibody MAC 445, rat anti-GABARAPL2 MAC446, and mouse anti-β-actin monoclonal antibody AC-15 respectively, followed by the corresponding HRP-conjugated second antibodies) by Western blotting. The experiment was performed on both clones 5 and 6 from panel B with similar results – that from clone 6 is shown. D) A myc-GIMAP6 T-Rex HeLa cell line was incubated in the presence (plus) or absence (minus) of tetracycline for four days. Lysates were prepared, separated by SDS-PAGE, and Western blotted for the expression of GIMAP6 (using rat monoclonal antibody, MAC445), GABARAPL2 (rat monoclonal antibody, MAC446), or other Atg8 members and SQSTM1, using antibodies as detailed in the Materials and Methods section. The results shown are representative of two independent experiments. E) Total RNA was isolated from a myc-GIMAP6 T-Rex HeLa cell line which had been grown in the presence or absence of tetracycline for four days. qPCR was performed as described in the Materials and Methods section. Expression levels were normalised between samples to GAPDH and then the levels of individual RNA species represented as a fold-stimulation of the plus-tetracycline samples relative to those from cells maintained in the absence of tetracycline. Data are presented as mean ± range of two independent experiments. F) myc-GIMAP6 HEK293 cells (right-hand panels) or the corresponding vector cells (left-hand panels) were treated with 10 µM emetine for the indicated times. Cell lysates were prepared and analysed by SDS-PAGE and Western blotting using either anti-GABARAPL2 rat monoclonal MAC446 or anti-β-ACTIN followed by the appropriate HRP-conjugated secondary antibodies. The result shown is representative of two independent experiments. G) Individual clones of TRex-Jurkat cells carrying GIMAP6 shRNA sequences were either treated for 4 days with 1 µg/ml tetracycline or were similarly maintained but in the absence of tetracycline. Cell lysates were prepared and assayed for GIMAP6 (using mAb MAC445) GABARAPL2 (using mAb MAC446) or β-ACTIN expression. Clones 1-3 carried shRNA1 and clones 4-6, shRNA2 (see Materials and Methods). The gap between clones 2 and 3 reflects the removal of intermediate lanes because of inconsistent cell recovery in those samples.