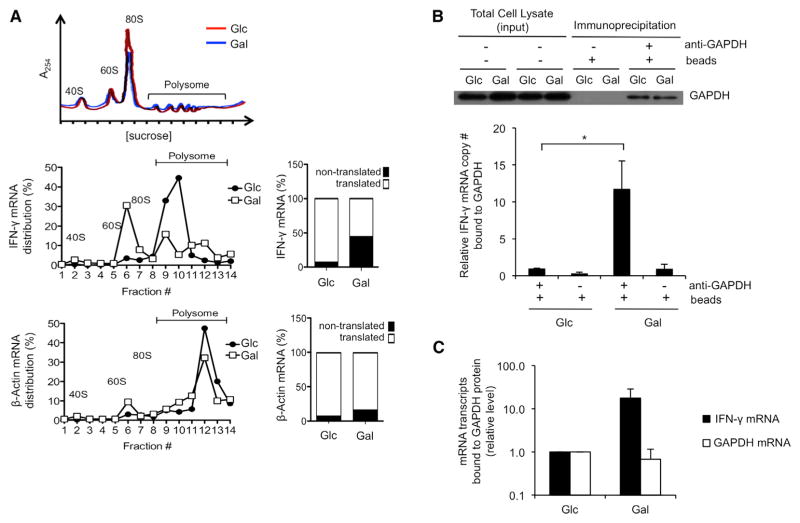
Figure 4. The Translational Defect in Cytokine Production Evident in the Absence of Aerobic Glycolysis Is Marked by Enhanced GAPDH Binding to IFN-γ mRNA.
(A) Polysome analysis of T cells that were activated with anti-CD3/28 for 3 days in media containing glucose and then differentially cultured in media with either glucose (Glc) or galactose (Gal) for an additional day. The 40, 60, and 80S ribosomal subunits and polysomes were fractionated and monitored with continuous A254 measurements. A representative polysome profile of cells in Glc (red) and in Gal (blue) is shown in the upper panel. Total RNA was extracted from each fraction, and IFN-γ (middle) and β-actin (bottom) expression was measured by qPCR and calculated as a percentage of total RNA collected in all fractions. The proportion of each mRNA between nontranslated (1–7) and translated (8–14) fractions is plotted. Data are representative results from two independent experiments.
(B) GAPDH-specific antibodies were used to immunoprecipitate GAPDH from extracts of activated T cells differentially cultured in media with either Glc or Gal. Bound GAPDH and associated mRNA were analyzed by western blot (top) and qRT-PCR (bottom). Total input per immunoprecipitation is shown (top). Data show results from six independent experiments as mean ± SEM (error bar) and are normalized to Glc cells (*p = 0.025).
(C) IFN-γ mRNA transcripts and housekeeping gene transcripts (GAPDH mRNA) that bind to GAPDH protein in cells cultured in galactose (Gal) versus cells cultured in glucose (Glc). Relative levels were calculated by subtracting background binding (no primary antibody control) from primary antibody-specific binding and then normalizing to transcripts levels bound to GAPDH in Glc cells.
Mean ± SEM (error bar) of three independent experiments. See also Figure S4.