Abstract
Introduction:
Hospital-acquired Urinary tract infections make 35% of all the hospital-acquired infections, and about 80% of them are related to the catheterization of the urinary bladder
Purpose:
To determine clinical characteristics and dominant etiologic factors of Urinary Tract Infections associated with urinary catheter (C-UTIs).
Methods:
Determined clinical characteristics of C-UTIs were prospectively analyzed on 38 hospitalized patients in the Clinic for Infectious Diseases at the University Clinical Centre Tuzla, from January 1st 2011 to December 31st 2011. The control group constituted of 200 patients with community-acquired Urinary Tract Infections (Co-UTIs) hospitalized in the same period.
Results:
It was registered on 22 (57.89%) of symptomatic infections, 14 (36.84%) asymptomatic bacteriuria and 2 (5.26%) other C-UTIs. Dominant etiologic factors were: E. coli, caused 14 (36.84%), Extended-Spectrum Beta-lactamase producing (ESBL) Klebsiella pneumoniae 7 (18.42%), Enterococcus faecium and Candida spp. 3 (7.89%) of C-UTIs. E. coli was significantly most common etiologic factor of C-UTIs in younger women (p=0.04). E. coli from C-UTIS showed significantly higher resistance to antimicrobial drugs. Inadequate antimicrobial therapy was significantly more common prescribed to patients from C-UTIs. Lethal outcome was significantly most common associated with certain clinical and laboratory findings.
Conclusion:
Initial antimicrobial therapy of those serious infections should be based on data from those research.
Key words: Urinary tract, Infections, Catheter.
1. INTRODUCTION
Hospital-acquired Urinary Tract Infections (H UTI) present the UTI-s acquired in the hospital environment at least forty eight hours after the patient admission. It usually appears as a consequence of the invasive diagnostic and therapeutic treatments of the urinary tract (1). Those infections make 35% of all the hospital-acquired infections, and about 80% of them are associated to the catheterization of the urinary bladder, which is why they are also often named as a catheter-associated UTI (C-UTIs).
Urinary tract catheterization increases the risk of bacteriuria, because of the bacteria’s tendency to adhere to artificial materials. Intraluminal infection direction is found in 23% of the C-UTI, as a consequence of the reflux of a numerous bacteria from the drainage bag. Extra luminal infection direction usually appears during the catheter placement, when bacteria from the periurethal area, or from the staff hands, impresses into the urinary bladder (3), or it can appear later when the bacteria migrates within the space between the catheter and urethral mucosa, which has been found in 35% of the patients (4). Prevalence of the infection increases with the catheterization period, if the catheterization period is short, it appears with 10-50% of the patients, and when the catheterization period is longer it appears with practically all the patients (5).
Only eight hours after the catheterization process, the inner and outer catheter surfaces contain a biofilm with bacterial cells, their polysaccharide glycocalyx, Tamm-Horfsfall proteins, struvite and apatite crystals and fibrin exudates of the damaged cells. Bacterias most frequently isolated within the biofilm are: Staphylococcus epidermidis, Enterococcus faecalis, E. coli, Proteus mirabilis, Pseudomonas aeruginosa and fungi (6). Uropathogenic E. coli which belongs to the fimbriae Type 3, is more frequently present in the biofilm (7). Bacteria in the biofilm change their phenotype, their cell involucre structure is different from other bacteria, their procreation is slower, antimicrobial therapy cannot effect them and the patient cannot defend from them (8).
Antimicrobial resistance of the etiologic factors related to this infection, continuously increases, and it is different in some parts of the world, in some parts of one country, even in different sections of one hospital. However, those general recommendations for a their treatment should be in accordance with the local situation (9). Taking into account all the information previously mentioned the aim of those study was to analyze clinical characteristics and dominant etiologic factors of C-UTIs in the Clinic for Infectious Diseases Tuzla.
2. PATIENTS AND METHODS
The research was conducted as a prospective study, which included 38 medically treated patients in the Clinic for Infectious Diseases Tuzla, in the period between 1st July 2010 and 31st December 2010. All the patients acquired the UTI during the hospitalization, and the infection was associated to the urinary catheter placement. Control group consisted 200 patients who acquired the UTIs outside the hospital (community-acquired UTIs, Co-UTIs) and were medically treated in the Clinic for Infectious Diseases Tuzla in the same period. The urine for microbiological examination was taken from the urine catheter using the classical procedure, following all the sepsis and antisepsis principles. Identification of the etiological factors for UTI was completed using standard microbiological methods.
Antibiogram was conducted using the classical disc-diffusion method on the Muller- Hinton agar for the following antimicrobial drug: penicillin, ampicillin, amoxicilline, co-amoxiclav, piperacillin, ciprofloxacin, norfloxacin, imipenem, meropenem, vankomycin, cephuroxime, cephotaxime, cephtriaxone, ceftazidime, cefepime, erythromycin, doxycycline, gentamicin, amikacin, co-trimoxazole and nitrofurantoin. This antimicrobial resistance analysis included resistant or sensitive isolates only, while those intermediary ones were excluded from the analysis. Assessment of the antimicrobial resistance was determined by the ratio of the resistant and tested number of isolates.
Biochemical analysis of blood and urine was conducted by using standard biochemical methods. UTIs diagnosis was set according to the medical history of the patients, taken from them personally, taken after the physical examination, biochemical and microbiological urine and blood test, echosonographic test of the urinary system, and other necessary radiologic and endoscopic examinations. Prevalence analysis of the C-UTI’s etiological factors and their antimicrobial resistance, was completed according to the sex and age of the patients, since all these demographic characteristics affect them. The patients were divided into two groups according to their age: younger age group (to 65 years old) and older age group (over 65 years old).
Statistical methods
T-test of proportions difference was used for examining the statistical significance of the samples’ proportions difference, while the nonparametric X2 test was used for testing the importance of distribution differences in some samples. Connection between the data was established according to the calculation of the correlation coefficient and the corresponding significances, using the Pearson’s correlation. The hypotheses testing reached the presumed significance level of 5% (p=0.05) in all calculations.
3. RESULTS
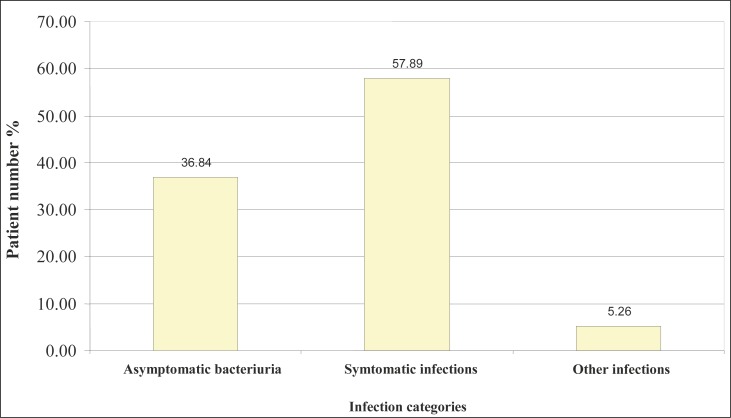
Symptomatic infections were the most frequently registered with the patients who have the C-UTIs, it was registered with 22 patients (57.89%). Asymptomatic bacteriuria was registered in 14 patients (36.84%), while 2 patients (5.26) were registered for other infections (Figure 1).
Figure 1.
Category of catheter-associated Urinary Tract Infections.
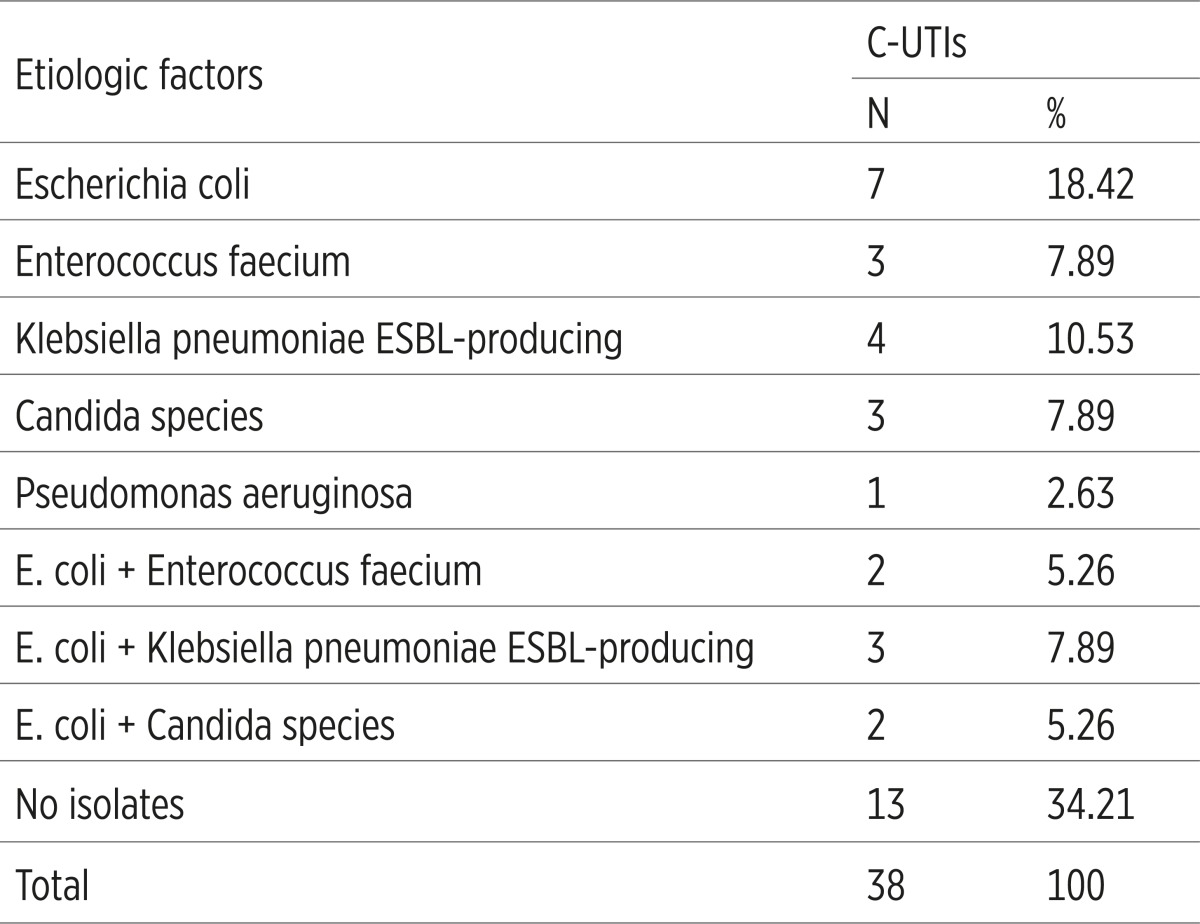
Dominant etiologic factors were set in the following order: E. coli caused 14 (36.84) infections, Extended-Spectrum Betalactamases-producing (ESBL) Klebsiella pneumoniae caused 7 (18.42%) infections, Enterococcus faecium and Candida spp. caused 5 (13.15%) C-ITUs (Table 1).
Table 1.
Etiologic factors of catheter-associated Urinary Tract Infections
 |
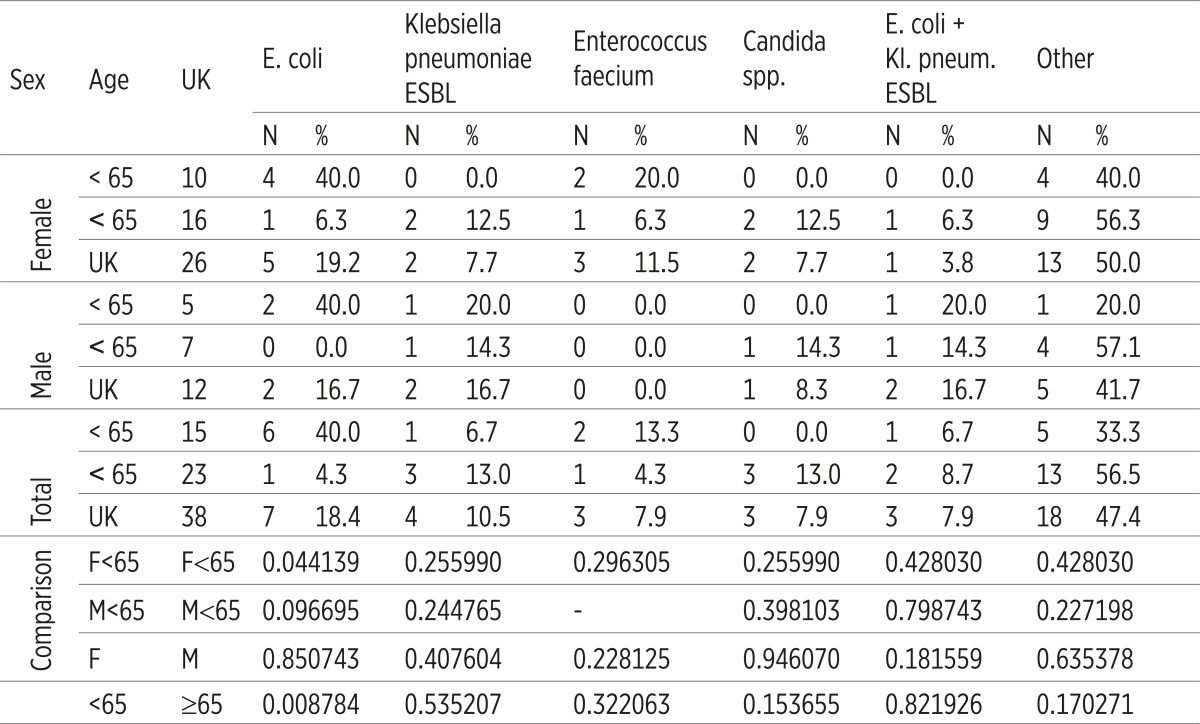
Statistically significant difference in the frequency of dominant etiologic factors according to sex was not registered. Enterococcus faecium was not registered as an etiologic factor of C-UTI in male. It has been established that, according to the age, E. coli was significantly more often as an etiologic factor in younger female (p=0.44139), as well as the total number of younger patients (p=0.008784). A significant difference in the frequency of other dominant etiologic factors related to sex and age of the patient has not been registered (p>0.05), (Table 2).
Table 2.
Age and sex distribution of dominant etiologic factors of catheter-associated Urinary Tract Infections
 |
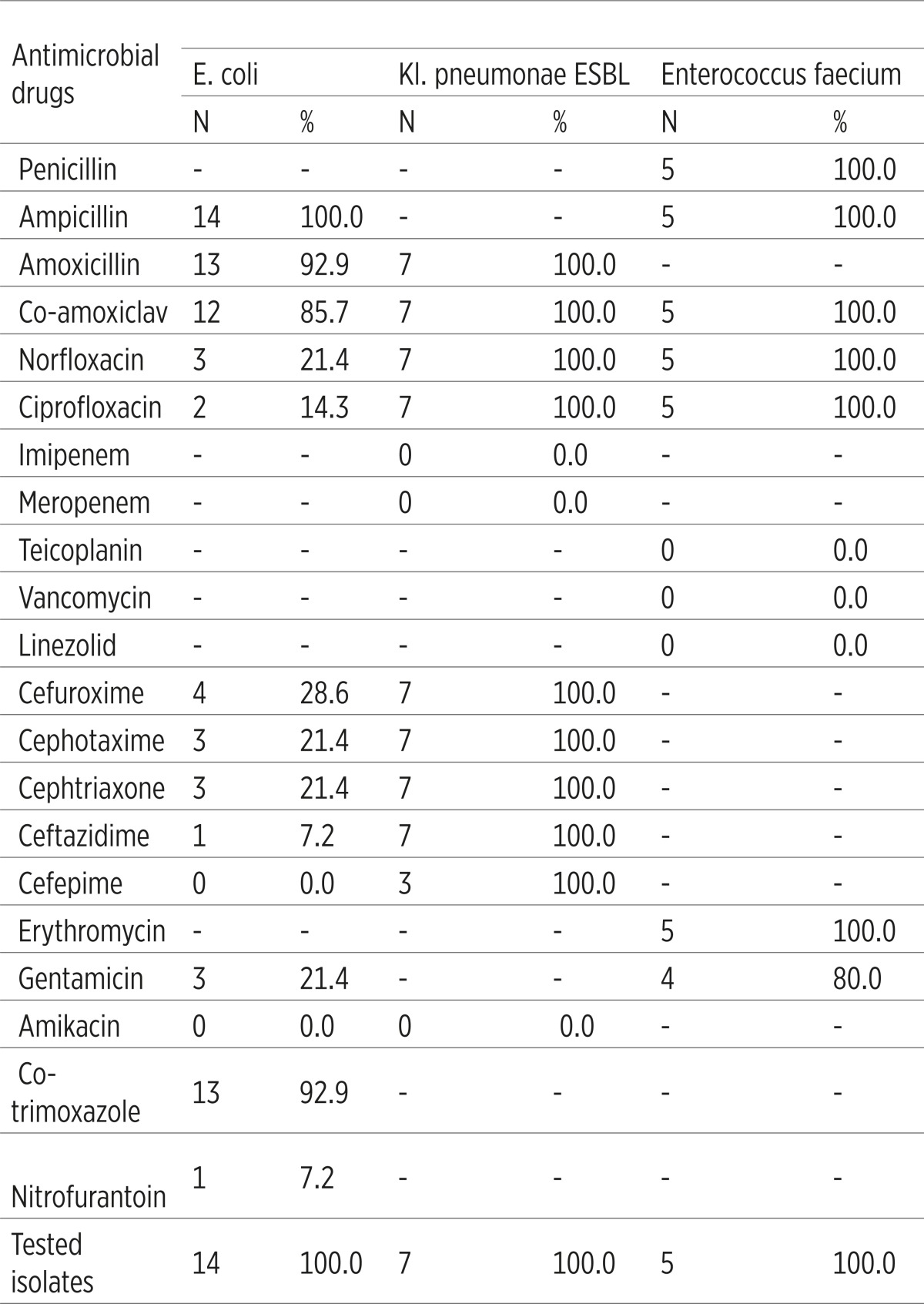
E. coli was not resistant to amikacin and cefepime (resistance 0%), its resistance to ceftazidime and nitrofurantoin was 7.2%, ciprofloxacin 14.3%, while the resistance to other antimicrobial drugs amounts 21.4-100%. ESBL Klebsiella pneumoniae was resistant to all the antimicrobial drugs except to imipenem, meropenem and amikacin (resistance 0%). Enterococcus faecium was not resistant to teikoplanin, vankomycin, and linezolid (resistance 0%), however it was 100% resistant to other antimicrobial drugs. When it comes to Candida albicans, antibiogram for it has not yet been conducted (Table 3).
Table 3.
Antimicrobial resistance of dominant etiologic factors of catheter-associated Urinary Tract Infections
 |
E. coli from the C-UTIs showed a significantly higher antimicrobial resistance to ampicillin, amoxicilline, co amoxiclav, cefuroxime, ceftriaxone, gentamicin and co trimoksazole (p<0.05), comparing to the E. coli from Co-UTis (Table 4).
Table 4.
Antimicrobial resistance of E. coli in community-acquired and catheter-associated Urinary Tract Infections
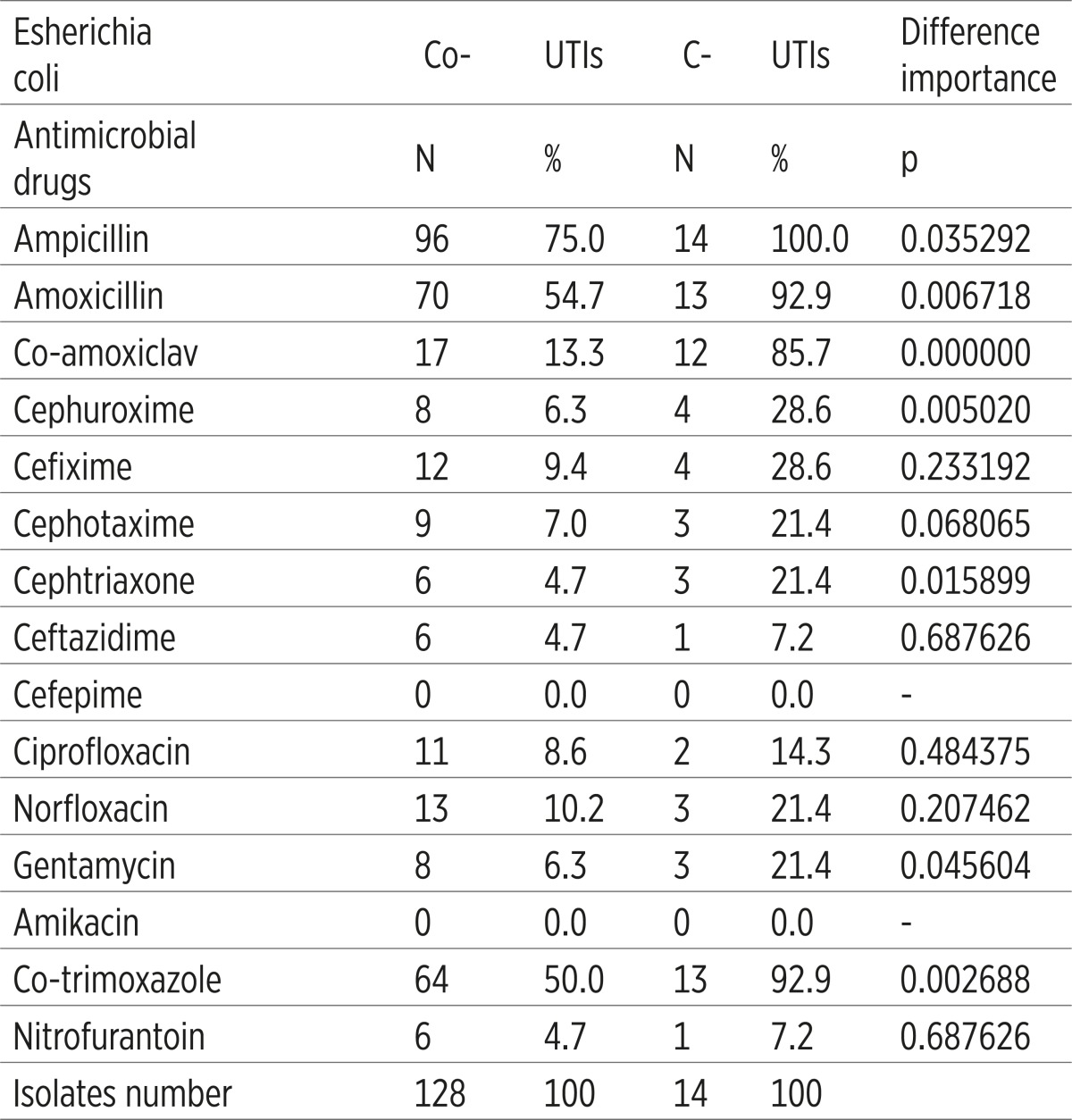 |
The adequate antimicrobial monotherapy and adequate combined antimicrobial therapy was applied significantly more common in the Co-UTIs, while the inadequate antimicrobial monotherapy and inadequate combined antimicrobial therapy were applied significantly more common in the C-UTIs, p<0.05,(Table 5).
Table 5.
Adequacy of subscribed antimicrobial therapy of community-acquired and catheter-associated Urinary Tract Infections
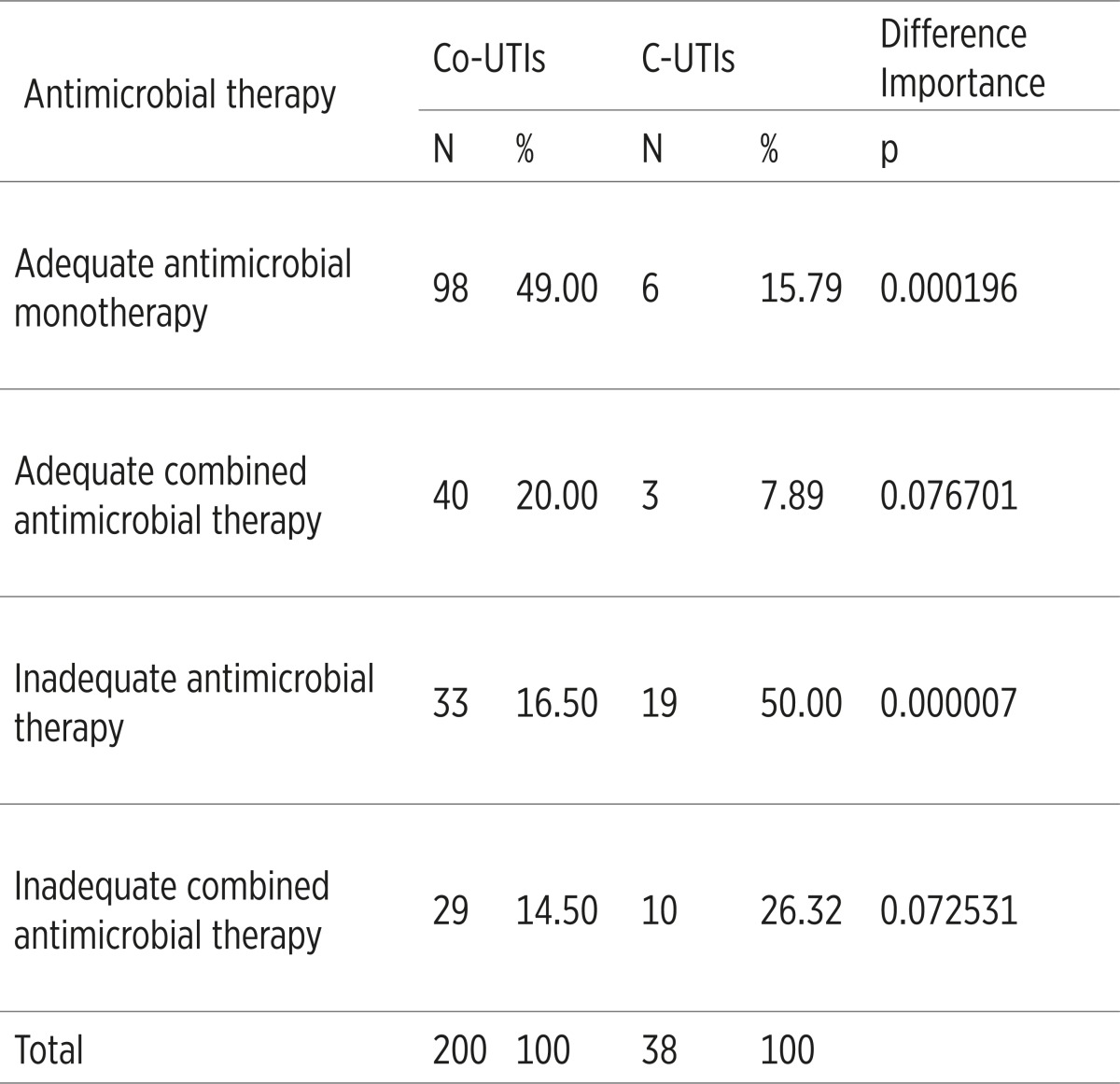 |
It has been established that certain clinic-laboratory parameters of the patient with C- UTIs correlate the lethal outcome. Risk factors such as diabetes, mellitus and other metabolic diseases (r=0.8287) as well as functional abnormalities of the urinary system (r=0.6642) considerably (p=0.000) correlate with the lethal outcome. According to the laboratory tests, it has been established that hypoproteinemia (r=0.7916), increased values of Lactat-dehidrogenase (r=0.7246), increased values of C-reactive protein (r=0.6299) and creatinine (r=0.5768) are in a significant correlation with the lethal outcome. When it comes to etiologic factors of the C-UTIs, Candida spp. significantly correlates with the lethal outcome (r=-0.4899; p=0.002), (Table 6).
Table 6.
Correlation risk factors, laboratory findings and etiologic factors of Urinary tract Infections with lethal outcome
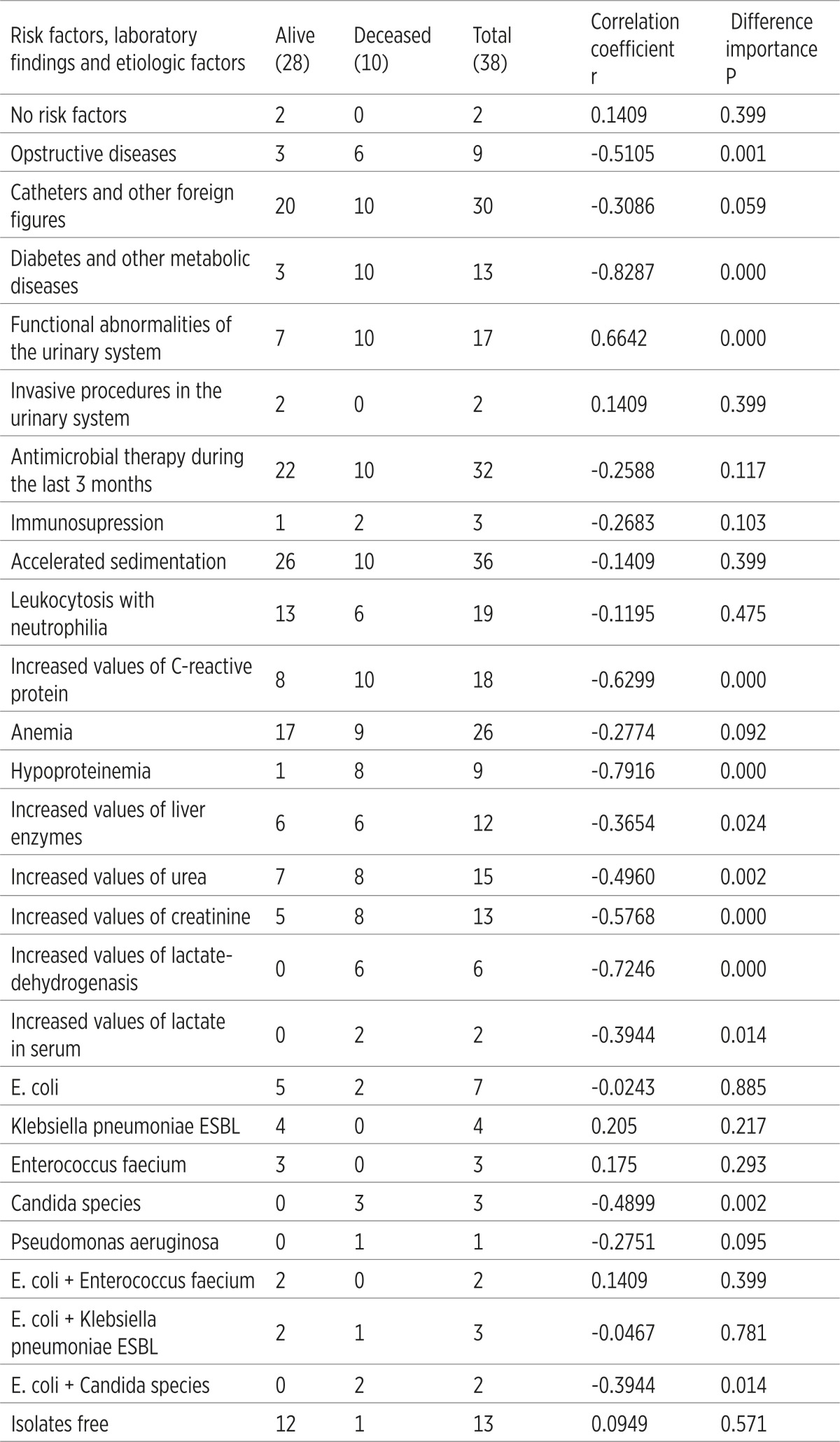 |
4. DISCUSSION
The most frequently present category of C-UTIs was the symptomatic C-UTIs, found in 22 of our patients (57.89%). Asymptomatic bacteriuria was found in 14 (36.84) patients. The results are in a close accordance with the results of other researchers, and they reported that, within the units of intensive care, 30% of the patients with urinary catheter had asymptomatic bacteriuria, 26% had the cystitis symptoms, and 20% of them had pyelonephritis symptoms (46% symptomatic UTIs) (10).
Prevalence of the C-UTIs etiologic factors is not the same in the whole world. It varies geographically and temporary. Amongst other factors it also depends on the age and sex of the patient, and it is necessary to continually evaluate the etiologic factors within some regions (9). Since patients in the Clinic for Infectious Diseases Tuzla come from all over the Tuzla canton, the results of the etiological factors prevalence, presented in this research, are applied to this region. Gram-negative bacteria still dominate the infections etiology, they are acquired in the hospital environment (11), but Candida species is more frequent, so the more adequate name for these infections is microburia, and not bacteriuria (2). Dominant etiologic factors of our patients, who suffer from C-UTIs, belonged to the Gram-negative bacteria, Gram-positive bacteria and fungus, Candida spp. E. coli caused 14 (36.84), Klebsiella pneumoniae caused 7 (18.42%) and Enterococcus faecium and Candida spp. caused 3 (7.89%) C-UTI-s. The research showed that, during the past 10 years, Candida spp. was more often being isolated as an etiologic factor of those infections, 20 to 44.4% (12, 13).
Analyzing the frequency of the C-UTIs dominant etiologic factors, there has not been registered a statistically significant difference in the frequency of dominant etiologic factors with regard to sex. Enterococcus faecium was not registered as an etiologic factorC-UTIs in the male. When it comes to age, it has been established that E. coli was a more frequent etiologic factor in the female of younger age.
The increase of antimicrobial resistance in some C-UTIs etiologic factors is worrying. E. coli as one of the dominant C-UTIs factors was not resistant to amikacin and cefepime (resistance 0%), resistance to ceftriaxone, ceftazidime and nitrofurantoin was estimated to 7.2%, to ciprofloxacin amounts 14.3%, while the resistance to other antimicrobial drugs was estimated to the value of 21.4-100%. E. coli resistance to the third generation of cephalosporins is in some countries estimated to the value of 10%, and 85-100% of this value is the ESBL-producing isolates. Resistance to fluoroquinolones is above 20% in most of the European countries (14).
ESBL Klebsiella pneumoniae is highly resistant to all antimicrobial drugs (42.9-100%), except to imipenem, meropenem and amikacin (resistance was 0%). Resistance to carbapenems is below 1% in most European countries, but in Greece it is estimated to the value of 43.5% (14).
Enterococcus faecium was not resistant to teiklopamin, vancomycin and linezolid, but it was 100% resistant to other antimicrobial drugs. Vancomycin-resistant isolates Enterococcus faecium reach the resistance percentage over 35% in Ireland and Luxemburg (14).
It was established that E. coli from the C-UTIs group in comparison to the E: coli from the CO-UTIs group, showed a significantly higher antimicrobial resistance to ampicillin, amoxicilline, co-amoxiclav, cefuroxime, ceftriaxone, gentamicin and co-trimoxazole. Results of other studies had shown that the hospital-acquired isolates are more resistant than the ones acquired outside of it (15, 16, 17), because these isolates are then joined with a more complicated UTI, longer antimicrobial therapy and the use of urinary catheter (2, 18).
Treatment of the C-UTIs is a special task, because it is often caused by multiresistant kinds of etiologic factors, so it is necessary to administer an adequate initial antimicrobial therapy which is in accordance with the information about their local antimicrobial resistance (9, 19). Inadequate antimicrobial therapy extends the treatment, and provides a possibility of complications, recurrence and the disease transformation into a chronic illness (9).
The results related to adequacy of the initial antimicrobial therapy, administered with our patients, were not so impressive. Inadequate antimicrobial monotherapy and inadequate combined antimicrobial therapy were administered with a total number of 29 (76.32%) patients suffering from C-UTIs, which underpins the fact that the assessment of possible etiologic factors and their antimicrobial resistance was not precise in most of the cases.
According to some researchers, results of a similarly designed study, conducted in France, were unsatisfactory, because they showed that the adequate antimicrobial therapy was administered only with 50.8% of cases, and the administration method was adequate in 94% of cases and dosed with 70.8% of cases. The authors of this research emphasize that it is important for doctors to follow local recommendations related to the treatment of UTI-s based on the local antimicrobial resistance (19).
Ten (26.32%) patients with C-UTIs suffered lethal outcome; all of them were elderly with background comorbidity. The results showed that all the 10 patients had a clinic-laboratory characteristics of urosepsis and septic shock. A statistical analysis of clinic-laboratory and etiologic factors established that some of them are significantly associated with the lethal outcome, and that they can be used to predict those consequences. When it comes to the risk factors, diabetes and other metabolic diseases and functional abnormalities of the urinary system, high values of C-reactive protein; hypoproteinemia, increased values of creatinine and increased levels of Lactat-dehidrogenase were significantly associated with the lethal outcome. Regarding the etiologic factors there was the Candida spp.
Results of similarly designed studies showed that the worse condition of the patient, longer hospitalization, low serum albumins, high LDH values (20), Candida spp. (21, 22) and MBL-producing kinds of Pseudomonas aeruginosa (23) are more frequently associated with mortality, and that an opportune adequate initial antimicrobial and supstitutional therapy, especially the adequate oxygenation, would significantly decrease mortality of patients (24).
5. CONCLUSION
According to this study the C-UTIs often had a negative outcome. The results of this research will help doctors how to choose an adequate initial antimicrobial therapy for those serious infections.
Conflict of interest
None declared.
REFERENCES
- 1.Horan TC, Emori TG. Definition of nosocomial infections. In: Abrytyn E, Goldmann DA, Scheckler WE, editors. Saunders Infection Control Reference Service. Philadelphia: W. B. Saunders; 1998. pp. 17–22. [Google Scholar]
- 2.Warren JW. Nosocomial urinary tract infections. In: Mandell GL, Bennet JE, Dolin RE, editors. Principles and practice of infectious diseases. 6th ed. Philadelphia: Churchill; 2005. pp. 3370–3380. [Google Scholar]
- 3.Maki DG, Tambyah PA. Engineering out the risk of infection with urinary catheters. Emerg Infect Dis. 2001;7:1–6. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tambyah PA, Halvorsen KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131–136. doi: 10.4065/74.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Donlan RM. Biofilms and device-associated infections. Centers for Diseaes Control and Prevention Atlanta, Georgia, USA, Special issue. 2001;7(2):277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trautner BW, Darouiche RO. Role of biofilm in catheter-associated urinary tract infections. AM J Infect Control. 2004;32:177–183. doi: 10.1016/j.ajic.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong CL, Ulett GC, Mabbett AN, Beatson SA, Webb RI, Monaghan W, et al. Identification of type 3 fimbriae in uropathogenic Escherichia coli reveals a role in biofilm formation. J Bacteriol. 2008;190(3):1054–1063. doi: 10.1128/JB.01523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stickler DJ, Morgan SD. Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheter. J Hosp Infect. 2008;69:350–360. doi: 10.1016/j.jhin.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Johansen TE. Nosocomially-acquired urinary tract infections in urology departments. Why an international prevalence study is need in urology. Int J Antimicrob Agents. 2004;23(1):30–34. doi: 10.1016/j.ijantimicag.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Baršić B. Bolničke infekcije mokraćnog sustava i urosepsa. Medicus. 2006;15(2):269–273. [Google Scholar]
- 11.Bouza E, San Yuan R, Munoz P, Voss A, Kluytmans J. A European on nosocomial urinary tract infections I. Report on the microbiology workload, etiology and antimicrobial susceptibility (ESGNI-003 study). European Study Group on Nosocomial Infections. Clin Microbiol Infect. 2001;7(10):523–531. doi: 10.1046/j.1198-743x.2001.00326.x. [DOI] [PubMed] [Google Scholar]
- 12.Laupland KB, Bagshaw SM, Gregson DB, Kirkpatrick AW, Ross T, Church DL. Intensive care unit-acquired urinary tract infections in a regional critical care system. Critical Care. 2005;9:60–65. doi: 10.1186/cc3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passos XS, Sales WS, Maciel PJ, Costa CR, Miranda KC, Lemos Jde A, Batista Mde A, Silva Mdo R. Candida colonization in intensive care unit patients’ urine. Mem Inst Oswaldo Cruz. 2005;100(8):925–928. doi: 10.1590/s0074-02762005000800016. [DOI] [PubMed] [Google Scholar]
- 14.Annonymous. Antimicrobial resistance surveillance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Available from: http://ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf .
- 15.Junquera S, Loza E, Baquero F. Changes in the antimicrobial susceptibility of Escherichia coli isolates from nosocomial versus community-acquired urinary tract infections. Enferm Infecc Microbiol Clin. 2005;34(4):197–201. doi: 10.1157/13073144. [DOI] [PubMed] [Google Scholar]
- 16.Lemort ML, Neuville S, Medus M, Gueudet P, Saada M, Aumaitre H, Lecaillon E. Comparative susceptibility evolution in Escherichia coli from urinary tract infections in outpatiens and inpatients at Perpignan hospital in 2002 and 2004. Pathol. 2006;54(9):427–430. doi: 10.1016/j.patbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Bean DC, Krahe D, Wareham DW. Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005-2006. Ann Clin Microbiol and Antimicrob. 2008;7:13–16. doi: 10.1186/1476-0711-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chulain MN, Murray AM, Corbett-Feeney G, Cormican M. Antimicrobial resistance in E. coli associated with urinary tract infection in the west of Ireland. Ir J Med Sci. 2005;174(4):6–9. doi: 10.1007/BF03168974. [DOI] [PubMed] [Google Scholar]
- 19.Saurel N, Pavese P, Boyer L, Vittoz JP, Decouchon C, Foroni L, et al. Adequacy of antibiotic therapy to guidelines for urinary tract infection in hospital. Med Mal Infect. 2006;36(7):369–374. doi: 10.1016/j.medmal.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Tal S, Guller V, Levi S, Bardenstein R, Berger D, Gurevich I, Gurevich A. Profile and prognosis of febrile elderly patients with bacteremic urinary tract infection. J Infect Dis. 2006;50(4):296–305. doi: 10.1016/j.jinf.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Lerma F, Nolla-Salas J, Leon C. Candiduria in critically ill patient admitted to intensive care medical units. Intensive Care Med. 2003;29:1069–1076. doi: 10.1007/s00134-003-1807-y. [DOI] [PubMed] [Google Scholar]
- 22.Bagshaw SM, Laupland KB. Epidemiology of intensive care unit-acquired urinary tract infections. Curr Opinion Infect Dis. 2006;(19):67–71. doi: 10.1097/01.qco.0000200292.37909.e0. [DOI] [PubMed] [Google Scholar]
- 23.Zawascki AP, Barth AL, Goncalves ALS. The influence of metallo-β lactamase production on mortality in nosocomial Pseudomonas aeruginosa infections. J Antimicrob Chemother. 2006;58:387–392. doi: 10.1093/jac/dkl239. [DOI] [PubMed] [Google Scholar]
- 24.Grabe M, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Lobel B, Naber K.G, et al. Guidelines on Urological Infections. European Association of Urology. 2009 [Google Scholar]