Table 2.
Gram-scale substrate scope of Mannich reaction.a
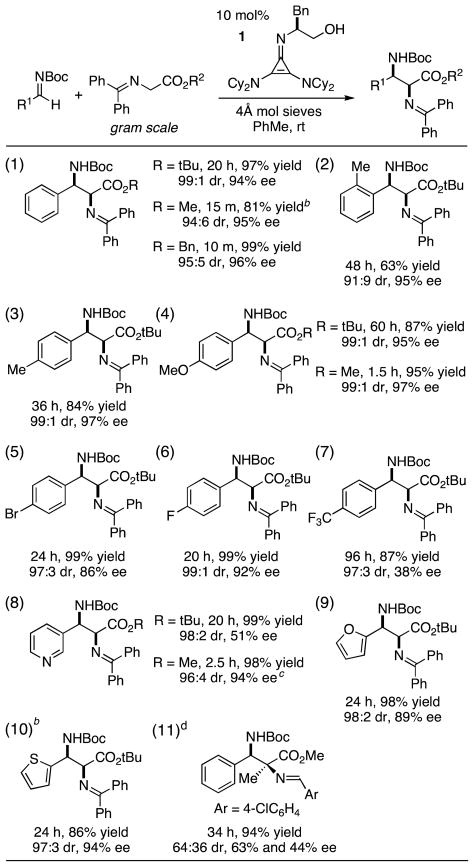
|
Yields based on purified products. Diastereomeric ratios (dr) and enantiomeric excesses (ee) were determined by HPLC.
Yield determined by 1H NMR versus Bn2O as a standard; product characterized after hydrolysis of the benzophenone imine.
Reaction performed at a concentration of 0.07 M.
20 mol% catalyst was used; 0.9 mmol scale.
