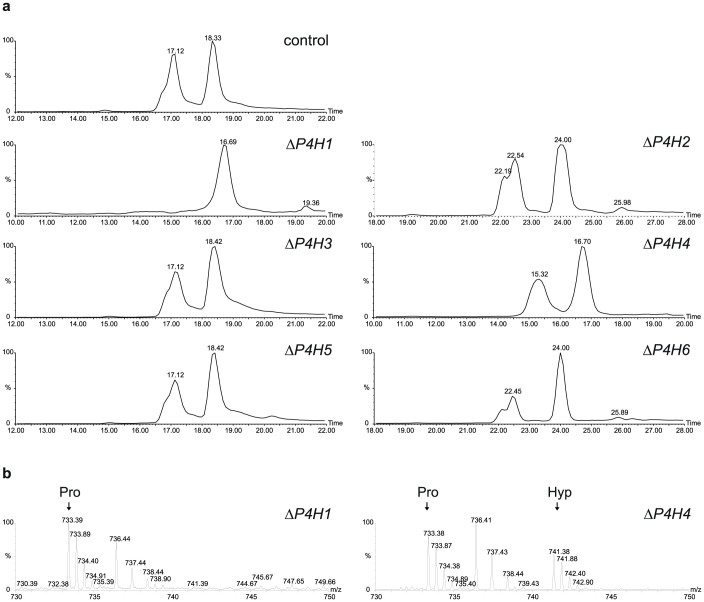
Figure 2. Mass spectrometric analysis of the hydroxylation of moss-produced rhEPO.
(a) Reversed-phase liquid chromatogram of tryptic peptides showing peaks of oxidized and non-oxidized peptide EAISPPDAASAAPLR (144–158) derived from rhEPO produced in moss lines 174.16 (control parental plant), ΔP4H1 #192, ΔP4H2 #6, ΔP4H3 #21, ΔP4H4 #95, ΔP4H5 #29 and ΔP4H6 #8. Selected ion chromatograms for the doubly charged ions of non-oxidized (m/z = 733.4) and oxidized peptide (m/z = 741.4) are shown. (b) Broad band sum spectra for peptide 144–158 showing the absence of prolyl-hydroxylation (Pro) in the line ΔP4H1 #192 and the presence of hydroxylated peptide (Hyp) in the line ΔP4H4 #95, as an example. The singly charged peak between “Pro” and “Hyp” is caused by the incidentally co-eluting peptide YLLEAK. Retention time deviations are technical artefacts.