Abstract
Objective
To evaluate the wound healing activity of the methanolic root extract of Buchanania lanzan Spreng. (B. lanzan), with a focus on antimicrobial and anti-biofilm properties.
Methods
The extract was evaluated for its wound healing properties (excision and incision models) as evident from the analysis of tensile strength and wound contraction. The extract was also screened for antibacterial properties against different Gram positive and Gram negative bacterial strains. B. lanzan was also studied for its effect on biofilm formation and disruption of preformed biofilms. The synergistic effect of B. lanzan was determined in combination with gentamicin.
Results
Topical application of B. lanzan (10% w/w ointment) significantly increased (40.84%) the tensile strength in the incision wound model. B. lanzan also showed significant wound healing activity in excision model and such significant activity was observed from the 9th day. Whereas Soframycin displayed significant wound healing activity from the 6th day. It was found that root extracts of B. lanzan revealed significant inhibition against all tested pathogens. B. lanzan displayed antimicrobial activity against Gram positive (MIC 0.625 mg/mL) and Gram negative (MIC 0.625–1.25 mg/mL). B. lanzan was able to reduce biofilm formation and also caused disruption of preformed biofilms in a manner similar to ciprofloxacin. However, gentamicin was found to be ineffective against biofilms formed by Gram negative organism. According to the fractional inhibitory concentration index, B. lanzan displayed synergistic activity when it was combined with gentamicin.
Conclusions
From this study it may be concluded that the root extract of B. lanzan revealed significant wound healing potential, which was supported and well correlated with pronounced antibacterial activity of the tested plant parts.
Keywords: Buchanania lanzan, Root and leaf extracts, Wound healing, Incision, Excision, Antibacterial, Antibiofilm
1. Introduction
Buchanania lanzan Spreng. (Chironji) (B. lanzan), a member of family Anacardiaceae, is a commercially useful tree found throughout the hot and dry deciduous forests of India[1]. Traditionally, the leaves are used for the management of wounds and also as a digestive, expectorant and purgative[2]. Some reports also indicate the usage of the plant as a cardiotonic, astringent and also for the treatment of skin diseases and glandular swelling[3]. The root is reported to be used as expectorant and also for curing blood diseases[4].
Pharmacological studies on B. lanzan indicate anti-inflammatory activity of the leaves[5], while the bark has been found to prevent cyclophosphamide induced genotoxicity and oxidative stress in mice[3]. Dry fruits of B. lanzan have been reported to show immunostimulant and astringent properties[6]. Kernel from the plant is known to posses antioxidant and anti-inflammatory activity[7]. The roots of the plant have been reported to to possess astringent properties and are also used in the management of diarrhoea. Phytochemical analysis of the plant reveals the presence of flavonoids, tannins, glycosides, phenols, steroid, saponin and gallic acid, and myricetin 3′-rhamnoside-3-galactoside has been detected in the leaves[8],[9].
According to literature, the process of wound healing involves several events (inflammation, proliferation and remodelling), which progress in an orderly fashion to restore the integrity of the damaged area. Immediately following an injury, the region is inflamed with release of various inflammatory mediators and then it is followed by fibroblast proliferation, angiogenesis and tissue remodelling[10],[11]. Various microbes are known to infect the wounds, resulting in a delay of the healing process.
The wounds are normally infected by microorganisms Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) (belonging to normal microflora) and also by others like Clostridium perfringes and Streptococcus pyogens[12]. There are many marketed antibiotics which are commonly used to overcome superficial wound infections by decreasing the microbial load[13],[14]. It has also been observed that infecting microorganisms are able to form biofilms on the wound surface, thereby leading to antibiotic resistance[15]–[18]. The biofilms also interfere with the immune system, facilitating the establishment of further bacterial communities which ultimately lead to the prolongation of the healing process[12],[19]. It is also interesting to note that plant derived products have been known to demonstrate synergy with synthetic products to combat different pathogens. Studies have also shown that several plant derived products with poor antimicrobial properties are known to display synergy against Gram negative bacteria when combined with different chemotherapeutic agents[20]–[22].
According to the survey of literature, there is a dearth of knowledge regarding the pharmacological properties of the roots of B. lanzan. Therefore, in the present investigation, we have tried to evaluate the wound healing property of the plant (root) with particular reference to microbial infections, effect on biofilm formation and evaluation of synergistic potential of the extract.
2. Materials and methods
2.1. Plant material and extract preparation
B. lanzan (Anacardiaceae) plants were procured from the forest area of Ranchi (Jharkhand), in the month of December and the plant was identified and authenticated from Central National Herbarium, Botanical Garden, Howrah, West Bengal [No.-CNH/I-I (81)/2005-Tech.II./1134]. The shade dried roots were crushed into small pieces and powdered. The powders of the roots were separately extracted in soxhlet extractor (250 g powders). Successive extractions with petroleum ether, ethyl acetate, chloroform and methanol were performed[23]. The methanolic root extract were concentrated under reduced pressure at 45–50 °C. The yield of B. lanzan was found to be 8.2%.
2.2. Phytochemical screening
Phytochemical screening was performed to identify the nature of the substances present in the different extracts. B. lanzan was tested for the presence of alkaloids, carbohydrates, flavonoids, steroids using different qualitative chemical methods[23].
2.3. Determination of total flavonoid content
About 0.5 mL of extract were separately mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminium chloride, 0.1 mL of potassium acetate and 2.8 mL of distilled water. After incubation at room temperature for 30 min, the absorbance was measured at 415 nm (UV-Visible spectrophotometer, Hitachi). The calibration curve was prepared with quercetin (concentration ranging from 1–50 µg/mL). Total flavonoid content was expressed in terms of milligram of quercetin equivalent (QE/g) of dried weight of the plant material[24].
2.4. Determination of total phenolic content
An aliquot (2 mL) of the extract was mixed with 1 mL of 1 mol/L Folin-Ciocalteu reagent. After 5 min, 4 mL of saturated sodium carbonate solution was added. The volume was then made up to 10 mL with distilled water and mixed thoroughly. The absorbance of the reaction mixtures was measured after a period of 2 h at 760 nm (UV-Visible spectrophotometer, Hitachi). Gallic acid was used to prepare the standard curve (0–50 mg/L). The results were expressed as milligram gallic acid equivalent (GAE/g) of dried weight of plant material[25].
2.5. Preparation of topical formulation
For assessment of excision and incision wound healing activity, the root extract were formulated in ointment base consisting of white soft paraffin, cetosteryl alcohol, paraffin and wool fat (16:1:1:1). The ointment contained 5% and 10% (w/w) B. lanzan, whereas 1% Soframycin ointment was used as the standard. The test samples and the standard were applied once daily.
2.6. Experimental animals
Wistar albino rats weighing (male; 150–200 g) were used in the study. Animals were procured from Laboratory Animal House of Birla Institute of Technology, Mesra. All animal experiments were strictly performed as per guidelines of the institutional animal ethical committee (No. 621/02/ac/CPCSEA). The animals were kept in polyacrylic cages and maintained under standard housing conditions of temperature (24–27 °C) and humidity (60%-65%) with 12 h:12 h light and dark cycles. They were acclimatized for seven days in the laboratory environment prior to the experimentation. Food was provided in the form of dry pellets and water ad libitum.
2.7. Acute dermal toxicity
The study was carried out to determine the therapeutic dose of the ointment of methanolic extract. The acute skin toxicity testing of the methanolic extract was done by applying 2 g of the ointment containing the extract (10% w/w) on the shaved dorsal region of the rat and observing the skin for irritation and inflammation for a period of seven days.
2.8. Wound healing activity
2.8.1. Circular Excision wound model
Animals, six rats in each group, were anesthetized with light ether and a circular piece of the skin (300 mm2 in area) was excised from the dorsal interscapular region. Wound contraction was monitored by measuring wound area, planimetrically, every three days till the wounds were completely healed[26]. The excision wound margins were determined at three days interval and the measurements were continued up to fifteen days. The wound contraction percentage was determined as follows[27].
Percent wound contraction = (healed area / total wound area) × 100
2.8.2. Linear incision wound model
Rats were anesthetized with light ether and then two para-vertebral straight incisions of 2 cm length were made through the entire thickness of the skin, on either side of the vertebral column with the help of a sharp scalpel[28]. After complete homeostasis the wounds were closed by means of interrupted sutures placed at equidistant points (1 cm apart). On the 7th day, sutures were removed and on the 10th post-wounding day, the tensile strength was measured by continuous water flow technique[29].
2.8.3. Histopathological examination
Sample tissues (excision wound model) were carefully isolated on the 10th day for each group of animals, for further histopathological examination. Tissues were fixed in 10% formalin and embedded in paraffin blocks. Thereafter the sections were stained with eosin and haemotoxylin, and were examined microscopically (40X; Leica) for keratinization, epithelization, fibrosis, mononuclear cells[30],[31].
2.9. Bacterial strains
The test microorganisms used in this study included both Gram positive [S. aureus MTCC 96 and Bacillus subtilis (B. subtilis) MTCC 441] and Gram-negative [E. coli MTCC 2939 and Pseudomonas aeruginosa (P. aeruginosa) MTCC 2453] bacteria. The organisms were cultured in fresh nutrient agar plates at 37 °C for 18–24 h. For antimicrobial testing, single colonies from the 24 h nutrient agar plates were used for inoculating an appropriate medium (5 mL). Cultures were grown aerobically at 37 °C with continuously shaking (100 r/min). Density of the broth (containing the suspended organisms) was adjusted to 0.5 McFarland standards with sterile distilled water[32]. The organism suspension was used within 30 min of the preparation.
2.10. Bacterial susceptibility assay
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using broth micro dilution method. Briefly, a standardized inoculum (10 µL of a 1–5×105 CFU/mL suspension) was added to the appropriate growth medium, supplemented with twofold serially diluted B. lanzan. About 0.1% DMSO was used as the negative control. After 16 h of incubation, the optical density was measured at 590 nm by Spectramex M5. The lowest concentration of the sample showing complete inhibition of bacterial growth was considered as the MIC.
For determination of MBC, the broth from the clear wells were spread on nutrient agar plates and incubated at 37 °C for 24 h. The minimum concentration of sample, at which the visual bacterial colony was reduced to 99.9%, was considered as the MBC.
2.11. Effect of B. lanzan on biofilm formation
Biofilm formation experiment was done using broth microdilution method. A standardized inoculum (5 µL of a 1-5×105 CFU/mL suspension) was inoculated with 100 µL of fresh Mueller Hinton broth in absence (non-treated control) and presence (treated) of B. lanzan. Ciprofloxacin and gentamicin were used as the positive controls. All the test agents were studied at sub-lethal concentration (at 1/2, 1/4, and 1/8MIC). After 16 h incubation, the plates were washed three times with phosphate buffer solution (pH 7.2) to remove the non adherent bacteria. The biofilm (slime and adherent cells) were fixed by incubating for 30 min (60 °C) and thereafter stained for 5 min at room temperature with 150 µL of 1% crystal violet solution. The bound crystal violet was eluted from the wells employing 150 µL of 33% glacial acetic acid for 15 min at 37 °C and then the OD was measured at 492 nm by Spectramex M5. The wells containing either the medium or the extract were used as blanks[33].
The percentage of inhibition of biofilm formation was determined as follows:
(1-OD at 492 of the test/OD at 492 of non-treated control)×100.
2.12. Effect of B. lanzan on preformed biofilm
A standardized inoculum (5 µL of a 1-5×105 CFU/mL suspension) was inoculated to 100 µL of Mueller Hinton broth, in each well of a U-bottom 96-well polystyrene microtiter plate. The plate was incubated for 24 h at 37 °C. The planktonic and poorly attached bacteria were removed by washing with 100 µL sterile broth. Preformed biofilms were then exposed to 100 µL of broth containing B. lanzan (prepared at 1, 5, and 10MIC). The plate was again incubated for 24 h at 37 °C. Following incubation, non-adherent cells were removed by washing with 300 µL sterile phosphate buffer solution (pH 7.2). The cells entrapped in the preformed biofilms were isolated by scraping with a pipette tip and subsequently exposed to 100 µL of 0.25% trypsin-EDTA for 5 min. The cell suspension was then vortexed and the bacterial counts were determined[33].
2.13. Checkerboard microdilution assay
Synergistic interactions between antibiotics (gentamicin) and B. lanzan was determined by the two-dimensional checkerboard microdilution assay, in 96-well microtitre plate using an 8-by-8 well configuration. Serial dilutions of B. lanzan and gentamicin ( 0.031 to 4MIC) were mixed together. After addition of a standardized inoculum (10 µL of a 1-5×105 CFU/mL suspension) the plate was incubated at 37 °C for 16 h. The growth in each well was quantified spectrophotometrically at 590 nm by a Spectramex M5. MICs for each combination of drugs were defined as the concentration of drug that reduced growth by 90% when compared the organisms grown in the absence of drug. All experiments were performed in triplicate. The fraction inhibitory concentration index (FICI) of each non-turbid well along the turbidity/non-turbidity interface was calculated by the method of Didry et al. as follows[34].
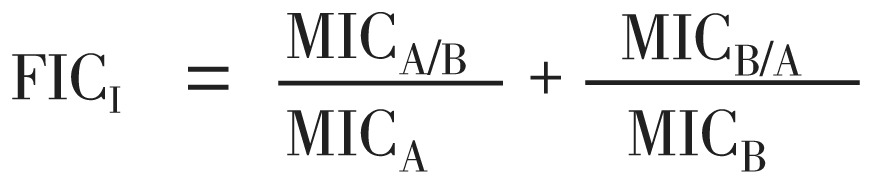 |
where MICA=MIC of the compound A alone and MICA/B is the MIC of compound A in combination with compound B. MICB and MICB/A are defined in the same way as for compound B. Synergism (FICI≤0.5), additive (0.5<FICI≤1), indifference (1<FICI≤2) or antagonism (FICI>2) between two compounds were deduced from the FICI.
2.14. Statistical analysis
The data on percentage wound healing was statistically analyzed using one-way analysis of variance (ANOVA) followed by Dunnet's test. The values of P<0.05 were considered statistically significant.
3. Results
3.1. Phytochemical analysis of the root extracts
Phytochemical analysis of the leaf and root extract revealed the presence of polyphenols, flavonoids with trace amounts of alkaloid and steroids.
3.2. Total flavonoid estimation
Total flavonoid content was expressed in terms of milligram of quercetin equivalent (QE/g) of dry plant material. B. lanzan were found to contain 335 mg QE/g of dry plant material.
3.3. Total phenolic estimation
Total phenolic content was expressed in terms of milligram of gallic acid equivalent (GAE/g) of dry plant material. In our study with B. lanzan, the total phenolic content was found to be 240 mg GAE/g of dry plant material.
3.4. Acute dermal toxicity
In the skin irritation study, the tested ointment did not show any type of irritation and there was no evidence of any noticeable inflammation on the skin.
3.5. Wound healing activity
3.5.1. Circular excision wound model
As shown in Table 1, 10% ointment of B. lanzan exhibited significant increase (73.28%) in wound contraction (from the 9th day onwards) as compared to the control. However better activity was shown by the standard drug Soframycin, which produced significant wound contraction from the 6th day.
Table 1. Effect of topical application of B. lanzan on excision wounds in rat.
| Group | Mean wound area (mm2) ± SEM (% contraction) |
|||||
| 0 day | 3rd day | 6th day | 9th day | 12th day | 15th day | |
| Vehicle control | 318.00±8.65 | 270.00±8.33 (15) | 213.00±7.18 (33.01) | 139.00±6.01 (56.28) | 80.00±5.10 (74.84) | 46.00±2.80 (85.53) |
| Soframycin (1%) | 303.00±8.39 | 238.00±8.58 (21.45) | 136.00±9.97 (55.11)** | 47.00±6.40 (84.48)** | 16.00±2.10 (94.71)** | 6.00±0.52 (98.01)* |
| B. lanzan (5%) | 314.00±8.55 | 272.00±7.76 (13.37) | 206.00±6.12 (34.39) | 129.00±7.71 (58.91) | 72.00±5.79 (77.07) | 35.00±3.50 (88.85) |
| B. lanzan (10%) | 307.00±10.14 | 268.00±8.09 (12.70) | 188.00±9.96 (38.76) | 82.00±7.51 (73.28)* | 38.00±6.78 (87.62)* | 14.00±2.68 (95.43)* |
Values are expressed as mean±SEM (% wound contraction), n=6, *P<0.05, **P<0.01 vs. control by one way-ANOVA followed by Dunnet's Test.
3.5.2. Linear incision wound model
In incision wound repair model (Table 2), water flow technique was used for determination of the tensile strength on the 10th day, following the linear skin incision. Topical application of 10% ointment of B. lanzan or the standard drug Soframycin improved the tensile strength significantly. However no significant imporovement could be observed with the 5% B. lanzan ointment.
Table 2. Effects of B. lanzan on incision wound model in rat.
| Serial No | Treatment | Tensile strength (g) Mean±SEM |
| 1 | Vehicle Control | 204.600±6.234 |
| 2 | Soframycin | 484.800±8.510** |
| 3 | B. lanzan (5%) | 211.600±4.900 |
| 4 | B. lanzan (10%) | 288.160±4.240* |
Values are expressed as Mean±SEM, n=6, *P<0.05, **P<0.01 vs. control by one way-ANOVA followed by Dunnet's Test.
3.5.3. Histopathological examination
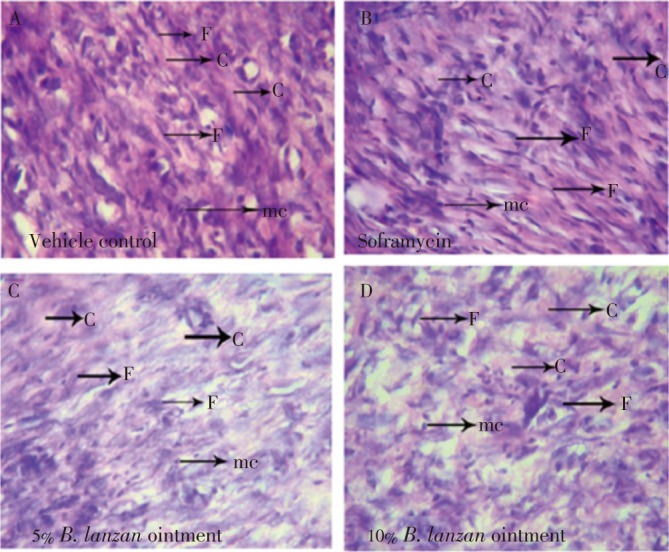
In haematoxylin and eosin stained sections (Figure 1), Soframycin and 10% B. lanzan group showed higher amount of fibroblast cells and collagen fibres including dermal blood vessel formation with mononuclear cell when compared to the control.
Figure 1. Histopathological view of wound healing and tissue remodelling in the vehicle control (A), Soframycin (B), 5% B. lanzan (C) and 10% B. lanzan (D) ointment. Skin sections were stained with haematoxylin and eosin (C: collagen, F: fibroblast, mc: mononuclear cells).
3.6. Anti microbial activity
3.6.1. MIC and MBC determination
The antibacterial activities were tested in vitro against both Gram negative and Gram positive strains (Table 3). B. lanzan inhibited Gram negative bacteria (E. coli and P. aeruginosa) at concentrations ranging from 0.625 to 1.25 mg/mL. In contrast, the MIC against Gram positive (S. aureus and B. subtilis) bacteria was found to be 0.625 mg/mL. As evident from the MBC values, B. lanzan demonstrated bactericidal activity against the different bacterial strains (Table 3).
Table 3. MIC and MBC for B. lanzan, ciprofloxacin (CIPRO) and gentamicin (GENTA) calculated for Gram positive (S. aureus and B. subtilis) and Gram negative (E.coli and P. aeruginosa) organisms.
| Organism |
B. lanzan |
CIPRO |
GENTA |
|||||
| MIC (mg/mL) | MBC (mg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |||
| S. aureus | 0.625 | 0.625 | 0.125 | 0.250 | 0.250 | 0.500 | ||
| B. subtilis | 0.625 | 0.125 | 0.250 | 0.500 | 0.125 | 0.250 | ||
| E. coli | 1.250 | 2.500 | 0.250 | 0.500 | 0.125 | 0.125 | ||
| P. aeruginosa | 0.625 | 1.250 | 0.125 | 0.250 | 0.0625 | 0.250 | ||
3.6.2. Effect of B. lanzan on biofilm formation
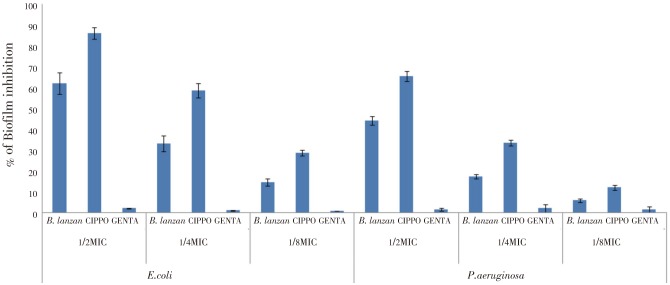
The effect of B. lanzan against biofilm formation by Gram negative organisms (E. coli, P. aeruginosa) was evaluated. The assay revealed a concentration-dependent reduction in biofilm formation (Figure 2). B. lanzan (1/2,1/4 and 1/8MIC) inhibited biofilm formation (62.18%±5.23%, 33.16%±3.84% and 14.56%±1.82% respectively) by E.coli, while (1/2 and 1/4MIC) concentrations of B. lanzan showed similar activity against P. aeruginosa, where the extent of inhibition was found to be 44.18%±2.07% and 17.4%±1.05% respectively. Standard drug gentamicin was found to be ineffective towards inhibition of biofilm formation, whereas ciprofloxacin displayed better inhibitory activity (1/2MIC-86.15%±2.88%; 1/4MIC-58.67%±3.41%; and 1/8MIC-28.60%±1.38%) when compared to B. lanzan.
Figure 2. Effect of B. lanzan, ciprofloxacin (CIPRO) and gentamicin (GENTA) at sub-MIC concentrations (1/2MIC, 1/4MIC and 1/8MIC) on biofilm formation of E.coli and P. aeruginosa. Biofilm formation was assessed by the colorimetric crystal violet-based technique. The results are expressed as percentage of biofilm inhibition with respect to untreated control. Values are expressed as Mean±SE.
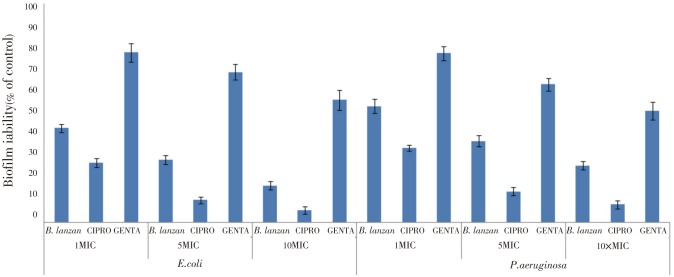
In addition, B. lanzan was also found to be very effective in disrupting the preformed biofilms produced by different Gram negative organisms (E. coli, P. aeruginosa). When the mature biofilms (E.coli) were treated with B. lanzan (1, 5, and 10MIC) the viable cell count decreased by 48.14%, 65.55%, and 79.81% respectively. In comparison, gentamicin (10MIC) and ciprofloxacin (10MIC) decreased the viable cell count by 32.73% and 93.22%, respectively (Figure 3).
Figure 3. Effect of B. lanzan, ciprofloxacin (CIPRO) and gentamicin (GENTA) at lethal concentrations (1MIC, 5MIC, and 10MIC) on preformed biofilm of E.coli and P. aeruginosa. The results are expressed as a percentage of the biofilm viability, assessed by colony counting, with respect to untreated control taken as 100%. Values are expressed as Mean±SE.
3.6.3. Synergy testing
The checkerboard microtiter plate assay was used to test the activities of B. lanzan in combination with gentamicin against S. aureus and E. coli (Table 4). The FICI was determined by the microdilution checkerboard assay model and form the results. It was observed that the combination of B. lanzan and gentamicin produced a synergistic response against both S. aureus and E. coli (FICI<0.5).
Table 4. In vitro interaction between B. lanzan and gentamicin determined by FICI.
| FICI |
Interpretation | ||
| Range | Mean FICI | ||
| S. aureus | 0.25 - 0.53125 | 0.3375 | SYN |
| E. coli | 0.25 - 0.53125 | 0.3875 | SYN |
SYN=synergy; Synergy was defined as an FICI of ≤0.5.
4. Discussion
The process of wound healing involves a complex sequence of events, and is initiated by an injury (a stimulus) to the tissues. These events commonly involve four phases: haemostasis, inflammation, proliferation and remodelling[35]. The substances which are known to accelerate the process of wound healing are generally referred as promoters of wound healing[36].
In the present investigation, the root extract (B. lanzan) was evaluated for its wound healing properties against excision and incision models of wound repair. Topical application of B. lanzan (10% w/w ointment) significantly enhanced wound healing process as observed in both excision and incision models. BLLE treatment increased the rate of wound contraction, thereby decreasing the time of healing when compared to control group of animals. It might be interesting to note that BLLE produced faster onset of the healing process and also reduced the time period for the complete healing to occur. The extract also facilitated the proliferation of epithelial cells after wounding (from wound edges), thus accelerating re-epithelialization and wound closure.
In our study, B. lanzan (10% w/w ointment) significantly increased the tensile strength of the wound when compared to the control. According to literature, increase in tensile strength of treated wounds probably results from increased collagen concentration and stabilization of the fibre[37]. Our observations are similar to that of reported findings on different plant extract which have shown beneficial properties on excision and incision wound models[38].
Histopathological evaluation of the tissues revealed an increase in the number of fibroblasts, collagen content, granulation and also the thickness of the scar tissue following treatment with 10% ointment of B. lanzan.
The above findings suggest a beneficial effect of the plant on different phases of wound healing which is actually reflected from the reduction of the healing time. The objective in wound healing management is to heal the wound in the shortest time frame, with minimum pain and discomfort to the patient.
According to reports, microorganisms are known to act as a barrier to the natural wound healing process. Wound infections are poly-microbial in nature, where some are known to be pathogen[12]. Poly-microbial nature of wounds was also studied[39], where analysis of 584 wounds from different sites revealed the presence of Staphylococcus and Clostiridium species[40].
In our investigation, B. lanzan was found to be active against both Gram positive (S. aureus and B. subtilis) and Gram negative bacteria (E. coli and P. aeruginosa). From our investigation it was observed that B. lanzan was more effective against S. aureus (commonly found in infected wounds). Moreover, the effectiveness of B. lanzan against both Gram positive and Gram negative organism would be important considering the polymicrobial nature of wounds.
As mentioned earlier, bacteria are known to form biofilms on attachment to different kind of surfaces[15]–[18]. Biofilm formation in wounds have been studied by different research groups and it has been found that S. aureus remains a common causative organism that has been found to be associated with biofilm formation in excisional wounds[41]. In another study with patient wound swabs, P. aeruginosa was predominant in the biofilms rather than planktonic forms[12]. Therefore, it can be said that bacteria colonizing chronic wounds exist as highly persistent biofilm communities. The evidence regarding the presence of bacterial biofilms in chronic wounds may be the primary reason for the delay associated with the healing process. Furthermore, presence of biofilm not only delays the process of healing (by imparting resistance to the action of the antibiotic) but also makes the region non responsive to the immune system[12]. Despite the fact that plant-derived antibacterial compounds generally demonstrate a low degree of activity, even though many plants have been found to display antimicrobial properties[42]. It may also be mentioned that polymicrobial nature of chronic wounds often requires a combination of different antibacterials (bacitracin/polymyxin B/neomycin; either double or triple topical combinations) to enhance the spectrum of activity. It may also be important to mention that such synergistic effects may actually help to decrease the effective dose of antibiotics on different resistant strains, thereby minimizing the side effects and simultaneously reducing the cost of the treatment. In the present investigation, B. lanzan was found to display a synergism when it was combined with gentamicin, and such synergic effect lead to an 8 fold decrease in the MIC of gentamicin. This reduction in the MIC of gentamicin by B. lanzan could be interesting, considering the toxicities associated with the use of different synthetic antibacterial and aminoglycosides in particular. However, it is difficult to predict the exact mechanism of such synergism. According to different researchers, the presence of natural products in such combinations may contribute towards: (i) bringing about structural change in the resistant bacteria, and facilitating the penetration of the drug through the outer layers of the bacterial cell wall; (ii) blocking the inhibitory effects of protective enzymes; (iii) interference with single or multiple metabolic targets of the antibiotic[42]–[44].
Therefore the effectiveness of B. lanzan in wound management coupled with the antibacterial and antibiofilm properties would be definitely interesting and may be promising in the management of wounds. Therefore the presence of these phytoconstituents (either alone or in combination) could be beneficial for augmenting the process of healing. Furthermore, the present findings validate the traditional claim on this plant. However, further studies are needed for the purification and characterisation of the active constituent(s) and subsequent understanding of the mechanism of action.
Acknowledgments
We are thankful to the University Grants Commission (DSA Phase-III and UPE-II programme; Dept. of Pharmaceutical Technology, Jadavpur University) for their support. We would also like to thank the authorities of the Department of Pharmaceutical Sciences, Birla Institute of Technology, Mesra (Ranchi) for their assistance.
Comments
Background
The polymicrobial nature of chronic wounds often requires a combination of different antibacterials (bacitracin/ polymyxin B/neomycin; either double or triple topical combinations) as a better treatment option enhancing increased spectrum of activity. The plant-derived antibacterial compounds generally exhibit antimicrobial properties. It may produce synergistic effects in combination with antibiotics.
Research frontiers
In this present investigation, the authors have designed to evaluate synergistic antibacterial activities of the methanolic root extract of B. lanzan, in combination with gentamicin in topical wound healing with a focus on anti-biofilm properties.
Related reports
Checkerboard microdilution assay, effect on biofilm formation, bacterial susceptibility assay, histopathological examination, linear incision wound model are reported. The methanolic root extract of B. lanzan, was evaluated through these different assay models and compared with standard antibiotic soframycin and ciprofloxacin.
Innovations and breakthroughs
B. lanzan is traditionally used for wound healing. This study was aimed towards evaluation of the wound healing activity of the methanolic root extract of the plant, with a focus on antimicrobial and anti-biofilm properties, and found significant activity of the plant part.
Applications
The effectiveness of B. lanzan in wound management coupled with the antibacterial and antibiofilm properties would be definitely interesting and may be promising in the management of wounds. It may also be important to mention that the synergistic effects in combination with gentamicin may help to decrease the effective dose of antibiotics on different resistant strains, thereby minimizing the side effects and simultaneously reducing the cost of the treatment.
Peer review
The authors have contributed a piece of excellent research work of traditionally used B. lanza root extract in the management of wound healing in a focus of antibacterial and biofilm properties through different bioassay models. The root extract of the plant in combination with gentamicin produced significant activities as antibacterial, wound healing and anti-biofilm properties.
Footnotes
Foundation Project: This work is supported by the University Grants Commission (DSA Phase-III and UPE-II programme; Dept. of Pharmaceutical Technology, Jadavpur University)
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Sastri BN. The wealth of India: raw material. New Delhi, India: CSIR; 1948. p. 233. [Google Scholar]
- 2.Kirtikar RK, Basu DB. Indian Medicinal Plants. Vol 3. Dehradun, India: International Book Distributor; 1935. [Google Scholar]
- 3.Jain R, Jain SK. Effect of Buchanania Lanzan Spreng. bark extract on cyclophosphamide induced genotoxicity and oxidative stress in mice. Asian Pac J Trop Med. 2012;5(3):187–191. doi: 10.1016/S1995-7645(12)60022-4. [DOI] [PubMed] [Google Scholar]
- 4.Rai MK. Review: biotechnological strategies for conservation of rare and endangered medicinal plants. Biodiversitas. 2010;11(3):157–166. [Google Scholar]
- 5.Mehta KS, Mukherjee S, Jaiprakash B. Anti-inflammatory activity of the methanolic extract of Buchanania Lanzan leaves by carrageenan-induced rat paw oedema method. Int J Pharm Sci Rev Res. 2011;6(2):144–146. [Google Scholar]
- 6.Puri A, Sahai R, Singh KL, Saxena RP, Tandon JS, Saxena KC. Immunostimulant activity of dry fruits and plant materials used in Indian traditional medical system for mothers after child birth and invalids. J Ethnopharmacol. 2000;71(1–2):89–92. doi: 10.1016/s0378-8741(99)00181-6. [DOI] [PubMed] [Google Scholar]
- 7.Warokar AS, Ghante MH, Duragkar NJ, Bhusari KP. Anti-inflammatory and antioxidant activities of methanolic extract of Buchanania Lanzan kernel. Indian J Pharm Educ Res. 2010;44(4):363–368. [Google Scholar]
- 8.Kumari A, Kakkar P. Screening of antioxidant potential of selected barks of Indian medicinal plants by multiple in vitro assays. Biomed Environ Sci. 2008;21(1):24–29. doi: 10.1016/S0895-3988(08)60003-3. [DOI] [PubMed] [Google Scholar]
- 9.Arya R, Babu V, Ilyas M, Nasim KT. Myricetin 3′-rhamnoside-3-galactoside from Buchanania lanzan (Anacardiaceae) Phytochemistry. 1992;31(7):2569–2570. [Google Scholar]
- 10.Clark RAF. Cutaneous wound repair. New York: Oxford University; 1991. p. 576. [Google Scholar]
- 11.Martin P. Wound healing- aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 12.Kirketerp-Moller K, Jensen PO, Fazli M, Madsen KG, Pedersen J, Moser C, et al. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46(8):2717–2722. doi: 10.1128/JCM.00501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobial for burn wound infection. Recent Pat Antiinfect Drug Discov. 2010;5(2):124–151. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh K, Anderson E, Harper JG. Overview and management of sternal wound infection. Semin Plast Surg. 2011;25(1):25–33. doi: 10.1055/s-0031-1275168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 16.Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 17.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 18.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 19.Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons S, Udo EE. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother Res. 2000;14(2):139–140. doi: 10.1002/(sici)1099-1573(200003)14:2<139::aid-ptr608>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Stavri M, Piddock LJ, Gibbons S. Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemoth. 2007;59(6):1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 22.Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46(10):3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harborne JB. Phytochemical methods. A guide to modern techniques of plant analysis. Plant Pathol. 1999;48(1):146. [Google Scholar]
- 24.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28(1):49–55. [Google Scholar]
- 26.Morton JJ, Malone MH. Evaluation of vulnerary activity by an open procedure in rats. Arch Int Pharmacodyn Ther. 1972;196(1):117–126. [PubMed] [Google Scholar]
- 27.Rashed AN, Afifi FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J Ethnopharmacol. 2003;88(2–3):131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich HP, Hunt TK. The effects of cortisone and anabolic steroids on the tensile strength of healing wounds. Ann Surg. 1969;170(2):203–206. doi: 10.1097/00000658-196908000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KH. Studies on mechanism of action of salicylates. II. Retardation of wound healing by aspirin. J Pharm Sci. 1968;57(6):1042–1043. doi: 10.1002/jps.2600570633. [DOI] [PubMed] [Google Scholar]
- 30.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid-ul Z. Healing potential of cream containing extract of Sphaeranthus indicius on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107(2):161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Taranalli AD, Kuppast IJ. Study of wound healing activity of seeds of Trigonella foeum graceum in rats. Indian J Pharm Sci. 1996;58:117–119. [Google Scholar]
- 32.Andrews JM. Determination of minimum inhibitory concentration. J Antimicrob Chemother. 2001;48(Suppl):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 33.Pompilio A, Scocchi M, Pomponio S, Guida F, Di Primio A, Fiscarelli E, et al. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides. 2011;32(9):1807–1814. doi: 10.1016/j.peptides.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Didry N, Dubreuil L, Pinkas M. Microbiological properties of protoanemonin isolated from Ranunculus bulbosus. Phytother Res. 1993;7(1):21–24. [Google Scholar]
- 35.Baie SH, Sheikh KA. The wound healing properties of Channa striatus-cetrimide cream-tensile strength measurement. J Ethnopharmacol. 2000;71(1–2):93–100. doi: 10.1016/s0378-8741(99)00184-1. [DOI] [PubMed] [Google Scholar]
- 36.Gurung S, Skalko-Basnet N. Wound healing properties of Carica papaya latex: in vivo evaluation in mice burn model. J Ethnopharmacol. 2009;121(2):338–341. doi: 10.1016/j.jep.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Akkol EK, Süntar I, Orhan IE, Keles H, Kanc A, Çoksari G. Assessment of dermal wound healing and in vitro antioxidant properties of Avena sativa L. J Cereal Sci. 2011;53(3):285–290. [Google Scholar]
- 38.Suguna L, Sivakumar P, Chandrakasan G. Effects of Centella asiatica extract on dermal wound healing in rats. Indian J Exp Biol. 1996;34(12):1208–1211. [PubMed] [Google Scholar]
- 39.Brook I, Frazier EH. Aerobic and anaerobic bacteriology of wounds and cutaneous abscesses. Arch Surg. 1990;125(11):1445–1451. doi: 10.1001/archsurg.1990.01410230039007. [DOI] [PubMed] [Google Scholar]
- 40.Brook I, Randolph JG. Aerobic and anaerobic bacterial flora of burns in children. J Trauma. 1981;21(4):313–318. doi: 10.1097/00005373-198104000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Davies CE, Wilson MJ, Hill KE, Stephens P, Hill CM, Harding KG, et al. Use of molecular techniques to study microbial diversity in the skin: chronic wounds reevaluated. Wound Repair Regen. 2001;9(5):332–340. doi: 10.1046/j.1524-475x.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 42.Aburjai T, Darwish RM, Al-Khalil S, Mahafzah A, Al-Abbadi A. Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Pseudomonas aeruginosa. J Ethnopharmacol. 2001;76(1):39–44. doi: 10.1016/s0378-8741(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 43.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Blaszyk M, Holley RA. Interaction of monolaurin, eugenol, and sodium citrate on growth of common meat spoilage and pathogenic organisms. Int J Food Microbiol. 1998;39(3):175–183. doi: 10.1016/s0168-1605(97)00134-7. [DOI] [PubMed] [Google Scholar]