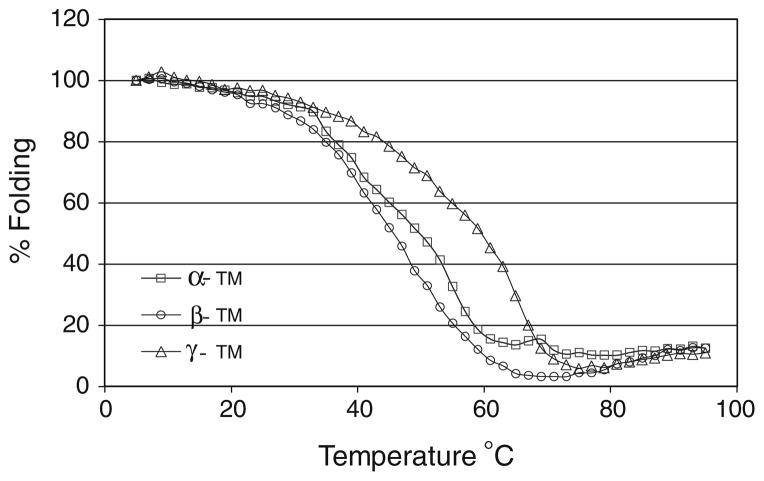
Fig. 6.
Thermal denaturation profiles of recombinant α-TM, β-TM and γ-TM TG homodimeric proteins assessed by circular dichroism analysis. The % folding of the proteins were plotted against the increasing temperature (°C). Fraction of the unfolded protein was determined based on the ellipticity at wavelength 222 nm. Results indicate the γ-TM TG homodimer is more stable than the α-TM homodimer, whereas β-TM is more flexible