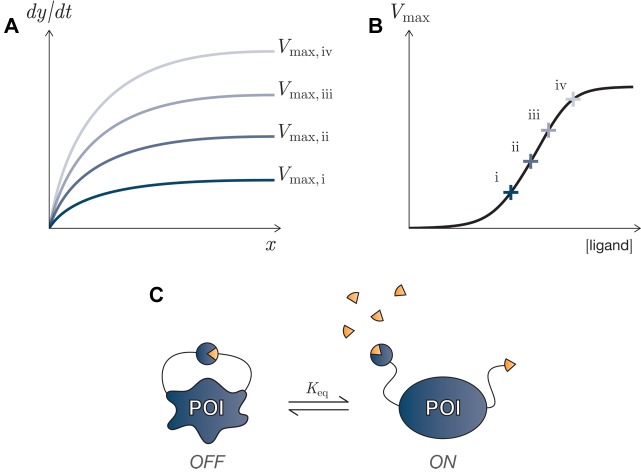
Figure 5.
Tuning of enzymatic reactions. (A) Michaelis-Menten kinetics for an enzyme-catalyzed reaction: the rate of the reaction x→y varies with the input concentration of the substrate, x, and with the enzymatic catalyst’s effective concentration and catalytic efficiency: Vmax = kcatE. (B) The relationship between Vmax and the input ligand can be characterized (and itself tuned): each marked point, i–iv, on the Vmax vs ligand concentration curve yields a different response curve shown in panel A. (C) A protein switch, in which the protein of interest (POI) contains autoinhibitory domains that bind and inactivate the enzyme’s catalytic activity, reducing the enzyme’s effective concentration. The presence of a competitively binding ligand can relieve the inhibition and restore catalytic activity.