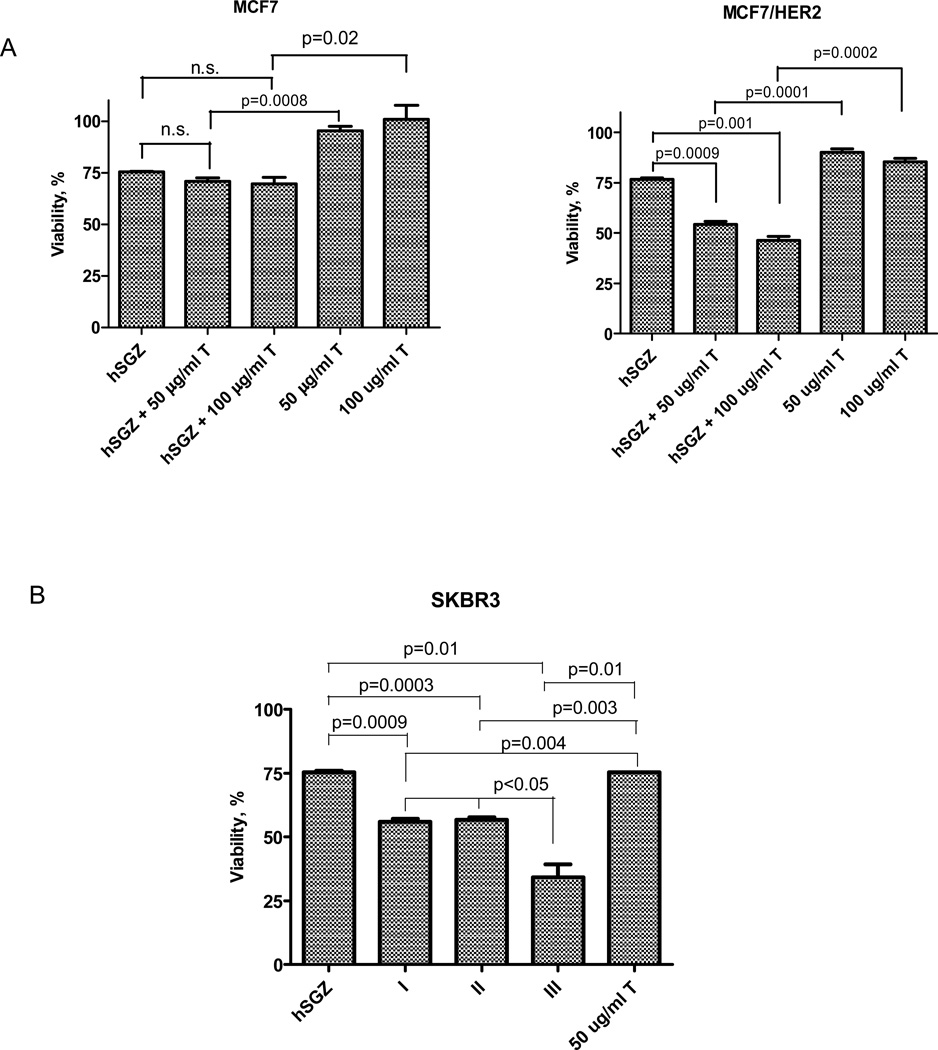
Figure 3.
Effect of hSGZ in combination with trastuzumab (T) in breast cancer cell lines. A, MCF-7 and MCF-7/HER2 cells were treated with hSGZ (at IC25 dose), with or without trastuzumab (50 or 100 µg/ml) for 72 h. B, SKBR3 cells were first treated with hSGZ (at IC25 dose) for 6 h, and then 50 µg/ml trastuzumab were added for 72 h (sequence I). Alternatively, cells were pretreated with 50 µg/ml trastuzumab for 6 h, followed by the addition of hSGZ (IC25). The cells were then incubated for a total of 72 h (sequence II). Coexposure hSGZ (at IC25 dose) and 50 µg/ml trastuzumab to the cells for 72 h (sequence III). Cell viability was assessed by crystal violet staining. Statistical significance was calculated using a two-tailed, independent samples t test. n.s., not significant (p>0.05).